Malignant hyperpyrexia multiplex PCR-LDR molecular diagnosis kit and application thereof
A technology of PCR-LDR and molecular diagnosis, which is applied in the field of multiple PCR-LDR molecular diagnosis kits for malignant hyperthermia, which can solve the problems of cumbersome operation, poor repeatability and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 149
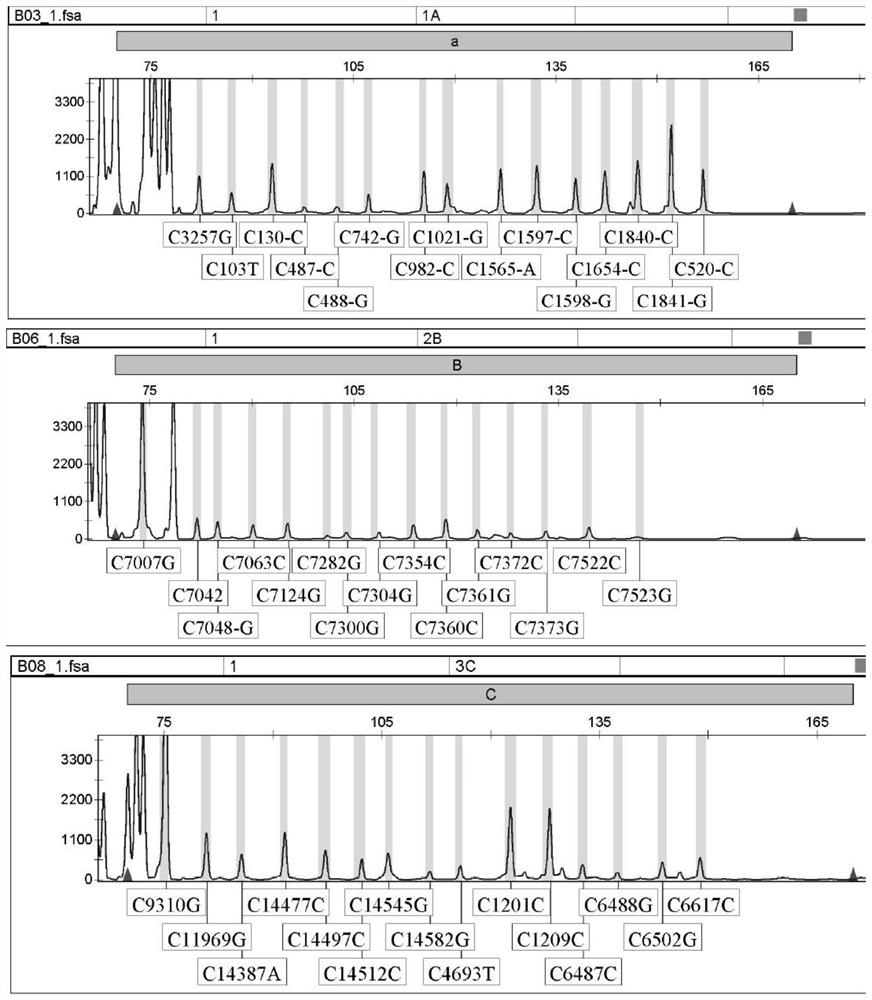
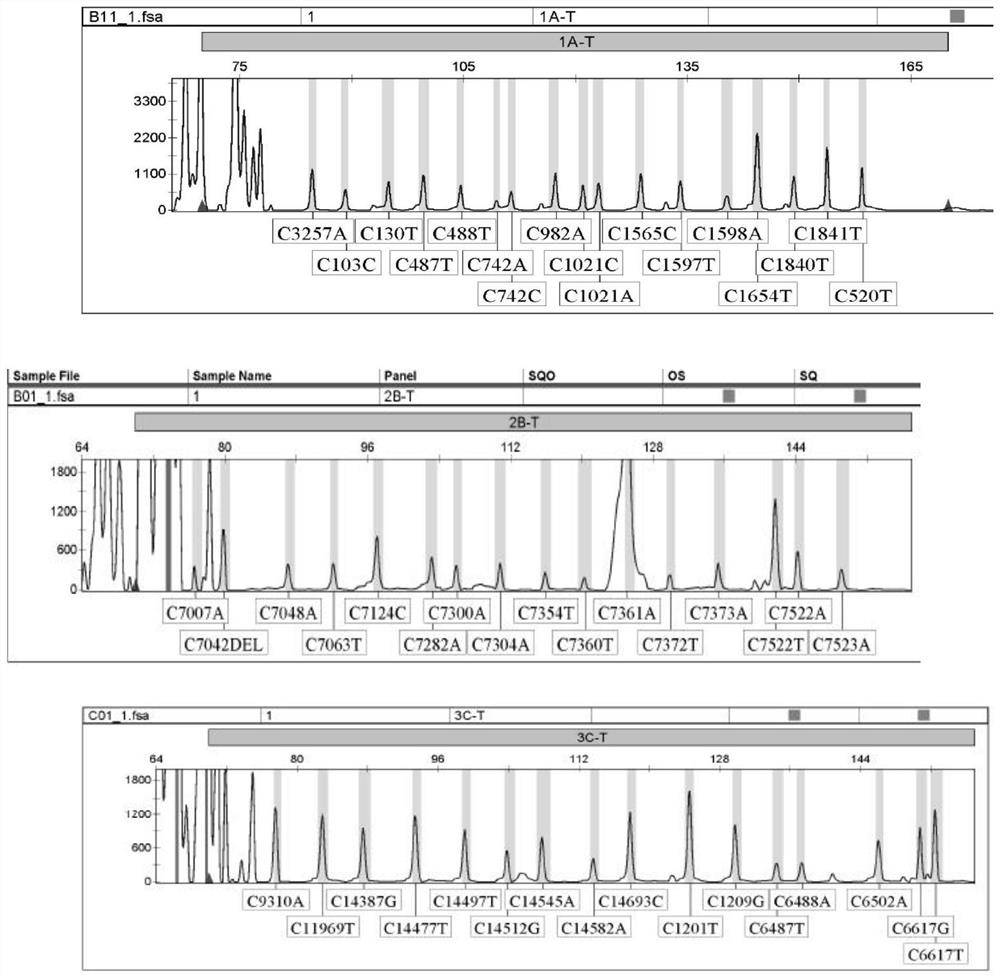
[0268] Example 1 PCR-LDR detection kit for 49 SNP sites and its use
[0269] 1. Kit composition:
[0270] PCR amplification reagents include: PCR buffer solution, MgCl2, the mixture Remi× of dNTPs, Ex-Taq enzyme, 18 pairs of primer mixtures (as shown in SEQ ID NO.1-36) and ultrapure water; LDR connection reaction reagent includes : 1 set of universal fluorescent probes and 49 sets of detection probe mixtures (as shown in SEQ ID NO.37-179), DNA ligase, 10× buffer and ultrapure water; genotyping reagents include: RO×-300 Internal standard, genotyping standards corresponding to 49 mutation sites used for genotyping; quality control products include: negative quality control product is sterilized double distilled water, wild type quality control product is 49 identified by sequencing The sites are all wild-type genomic DNA, and the positive quality control products are C.103T>C, C.130C>T, C.487C>T, C.488G>T, C.742G>A, C.742G>C , C.982C>T, C.1021G>C, C.1021G>A, C.1565A>C, C.1597C...
Embodiment 2
[0288] Embodiment 2 specificity test
[0289] Materials: 49 known positive mutant plasmid samples of SNP sites were taken, and 5 known negative samples were taken. All the above samples were verified by gene sequencing, and were tested with this kit at the same time. The results were analyzed and verified: 49 sites of PCR- The detection results of the LDR detection kit are completely consistent with the sequencing results, and the kit has strong specificity.
Embodiment 3
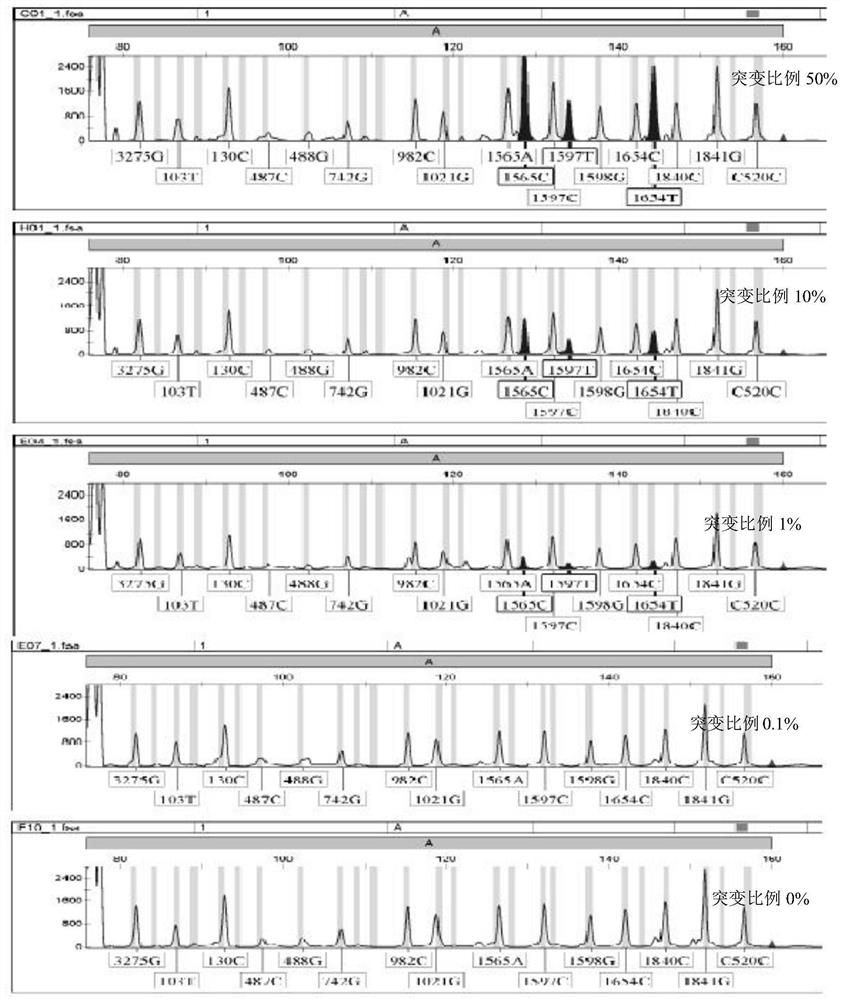
[0290] Example 3 Sensitivity test of mutation detection rate
[0291] With the wild-type mixed standard plasmid as the background, some mutant plasmids were added in proportions of 50%, 5%, 1%, 0.1%, and 0% to verify the sensitivity of mutation detection. The results show that the PCR-LDR-based 49 SNP site detection scheme can break through the limitations of traditional nucleic acid mutation detection technology, and detect multiple mutation sites with a detection sensitivity of up to 1%, see Figure 3A-C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com