Preparation method of 3-methyl-2-butenol
A technology of butenol and methyl, applied in the preparation of medicine and pharmaceutical intermediate 3-methyl-2-butenol, in the field of perfume, can solve the problem of accelerating catalyst system, the influence of reaction yield stability, and high synthesis cost problems, to achieve high industrial application value and environmental protection value, reduce the operation of solvent recovery and application, and improve the conversion rate and selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
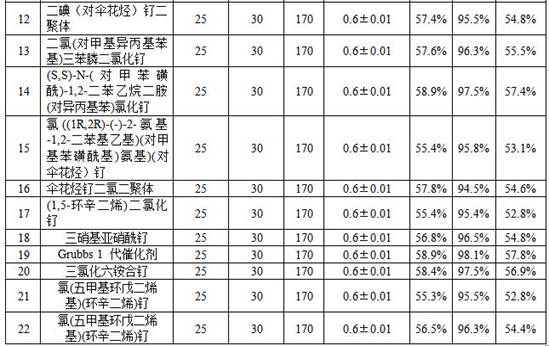
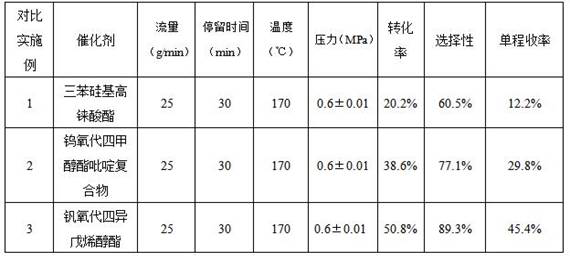
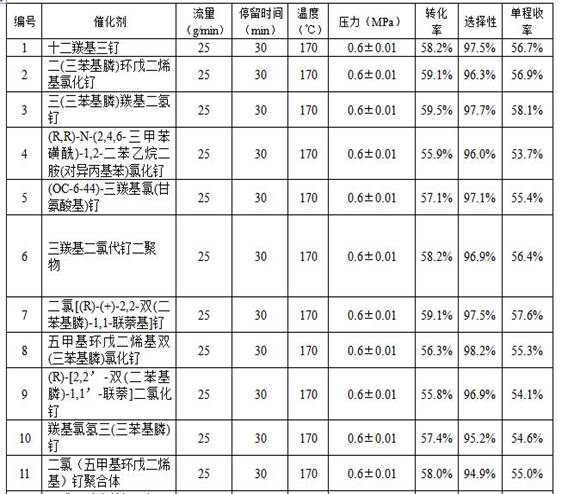
Image
Examples
Embodiment 1
[0033] Using a high-pressure metering pump, the mixed solution of 2-methyl-3-butene-2-ol and catalyst is pumped into the preheater at a constant rate of 25g / min, and the amount of catalyst is 2-methyl-3-butene- 0.02% of the mass of 2-alcohol, and at the same time set the temperature of the preheater to 130°C, preheat the reaction raw material 2-methyl-3-buten-2-ol and the catalyst to 130°C, and set the temperature of the tubular reactor to After the temperature of the tubular reactor is stabilized, adjust the reverse pressure controller to control the pressure at 0.6±0.01MPa, continuously feed and discharge the material, and the reaction residence time is 30min. After the reaction is completed, the reaction liquid is separated by rectification The crude product 3-methyl-2-butenol was obtained, and the reaction conversion was calculated to be 60.1%, and the selectivity was 98.5%.
[0034] The catalyst is composed of bis(triphenylphosphine)dicarbonylruthenium dichloride and tris...
Embodiment 2
[0036] Using a high-pressure metering pump, the mixed solution of 2-methyl-3-butene-2-ol and catalyst is pumped into the preheater at a constant rate of 25g / min, and the amount of catalyst is 2-methyl-3-butene- 0.02% of the mass of 2-alcohol, and at the same time set the temperature of the preheater to 120°C, preheat the reaction raw material 2-methyl-3-buten-2-ol and the catalyst to 120°C, and set the temperature of the tubular reactor to After the temperature of the tubular reactor is stabilized, adjust the reverse pressure controller to control the pressure at 0.5±0.01MPa, continuously feed and discharge the material, and the reaction residence time is 30min. After the reaction is completed, the reaction liquid is separated by rectification The crude product 3-methyl-2-butenol was obtained, and the reaction conversion rate was calculated to be 53.5%, and the selectivity was 97.5%.
[0037] The catalyst is composed of bis(triphenylphosphine)dicarbonylruthenium dichloride and...
Embodiment 3
[0039]Using a high-pressure metering pump, the mixed solution of 2-methyl-3-butene-2-ol and catalyst is pumped into the preheater at a constant rate of 25g / min, and the amount of catalyst is 2-methyl-3-butene- 0.02% of the mass of 2-alcohol, and at the same time set the temperature of the preheater to 140°C, preheat the reaction raw material 2-methyl-3-buten-2-ol and the catalyst to 140°C, and set the temperature of the tubular reactor to After the temperature of the tubular reactor is stabilized, adjust the reverse pressure controller to control the pressure at 0.8±0.01MPa, continuously feed and discharge the material, and the reaction residence time is 30min. After the reaction is completed, the reaction liquid is separated by rectification The crude product 3-methyl-2-butenol was obtained, and the reaction conversion rate was calculated to be 62.2%, and the selectivity was 94.2%.
[0040] The catalyst is composed of bis(triphenylphosphine)dicarbonylruthenium dichloride and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com