Light-cured resin based on organic polyatomic acid as well as preparation method and application of light-cured resin
A light-curing resin and polyacid technology, which is applied in the preparation of organic compounds, organic chemistry, and carboxylate ester preparation, etc., can solve the problems of decreased material properties, complex synthesis routes, and expensive raw materials, and achieves good remodeling performance. Simple process and the effect of saving oil resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Another aspect of the embodiments of the present invention provides a method for preparing a photocurable resin based on an organic polybasic acid, which includes:
[0037] Under solvent-free conditions, the homogeneous mixed reaction system comprising dibasic acid and / or tribasic acid, glycidyl methacrylate and epoxy ring-opening catalyst is heated to undergo a ring-opening reaction to obtain structures such as formula (I) and / or the photocurable resin based on organic polybasic acid shown in formula (II);
[0038]
[0039] Wherein, the dibasic acid has a structure shown in formula (i), and the tribasic acid has a structure shown in formula (ii):
[0040]
[0041] Wherein, R includes a substituted or unsubstituted aliphatic chain or an aromatic ring, wherein the substituent group contains more than one hydroxyl group.
[0042] In some preferred embodiments, the dibasic acid and / or tribasic acid containing carboxylic acid group raw materials are subjected to so...
Embodiment 1
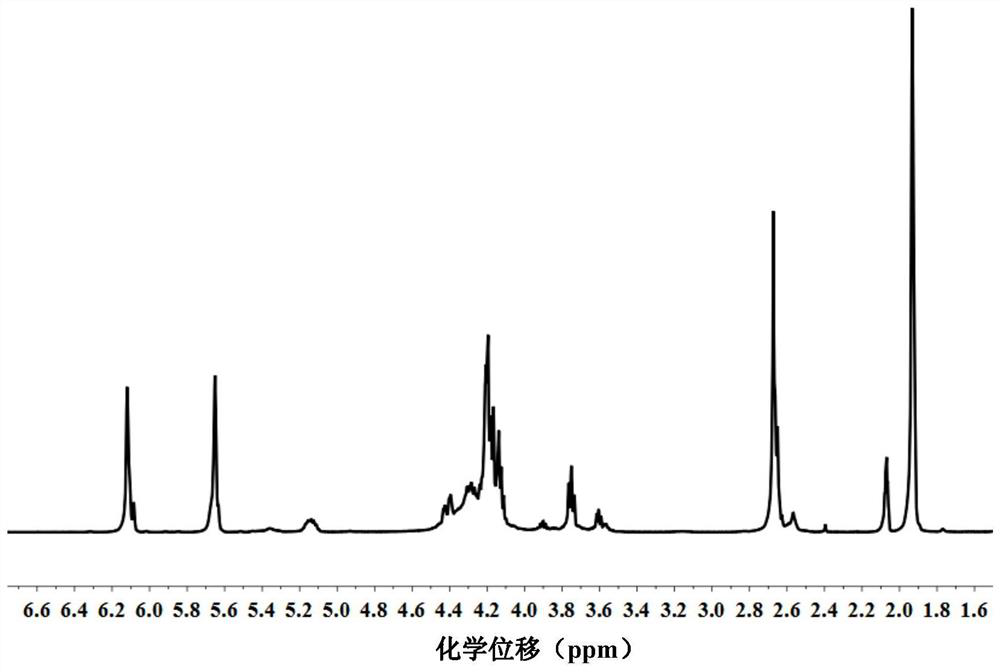
[0079] First, p-hydroxyanisole MEHQ (0.04g, 0.10wt%, based on the total weight of the mixture, the same below), antioxidant A71010 (0.04g, 0.10wt%) and triphenylphosphine TPP (1.24g, 10.0wt%) ). They were dissolved in glycidyl methacrylate GMA (28.4 g, 0.20 mol), added to a 500 ml four-necked flask equipped with nitrogen inlet, mechanical stirring, condenser and thermometer, and then heated to 60 °C for 1 h under nitrogen flow . Succinic acid (SA, 11.8 g, 0.10 mol) solid powder was added to the mixed system in batches at 95 °C at a molar ratio of 1:2 and GMA until clear and the temperature was kept at 100 °C for 3 h. Then the temperature was raised to 110° C. for 2 hours, and finally to 120° C. for 1 hour, and the resin was cooled to obtain a photocurable resin monomer, succinate diglyceride methacrylate (SG), with a yield of 100%. H NMR spectrum 1 H-NMR such as figure 1 As shown, there is a one-to-one correspondence between each peak on the figure and the atoms on the str...
Embodiment 2
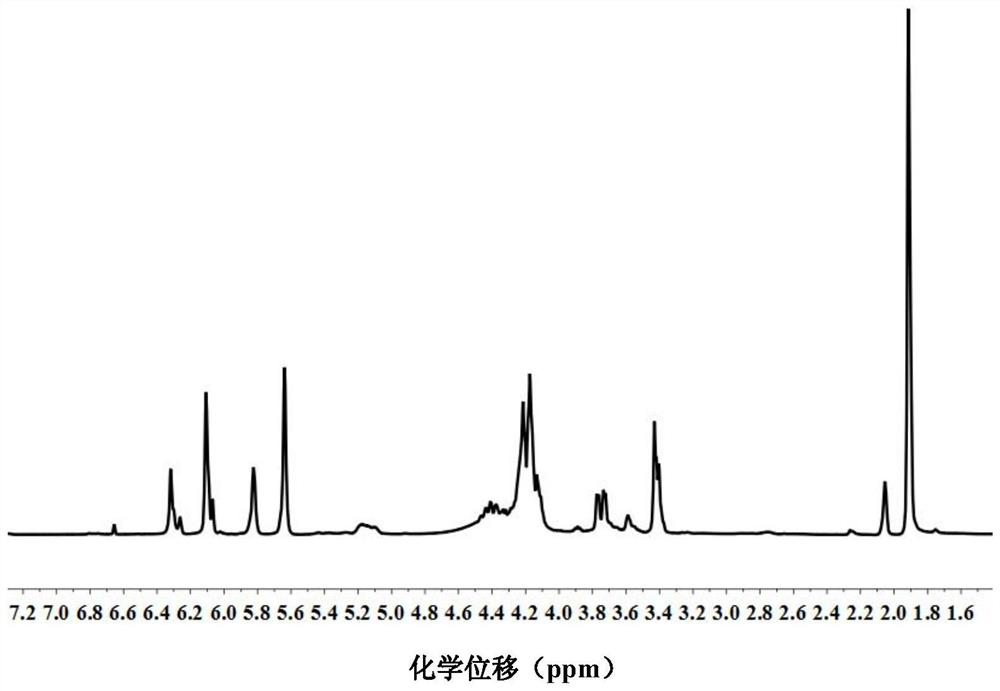
[0082] First, p-hydroxyanisole MEHQ (0.04g, 0.10wt%, based on the total weight of the mixture, the same below), antioxidant A71010 (0.04g, 0.10wt%) and triphenylphosphine TPP (2.07g, 5.0wt%) ). They were dissolved in glycidyl methacrylate GMA (28.4 g, 0.20 mol), added to a 500 ml four-necked flask equipped with nitrogen inlet, mechanical stirring, condenser and thermometer, and then heated to 60 °C for 1 h under nitrogen flow . Itaconic acid (IA, 13.0 g, 0.10 mol) solid powder was added to the mixed system in batches at 95 °C at a molar ratio of 1:2 and GMA until it became clear and the temperature was kept at 95 °C for 2 h. Then the temperature was raised to 105° C. for 1 h, and finally to 115° C. for 2 h, and the resin was cooled to obtain the photocurable resin monomer itaconate diglyceryl methacrylate (IG), with a yield of 100%. H NMR spectrum 1H-NMR such as figure 2 As shown, there is a one-to-one correspondence between each peak on the figure and the atoms on the st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com