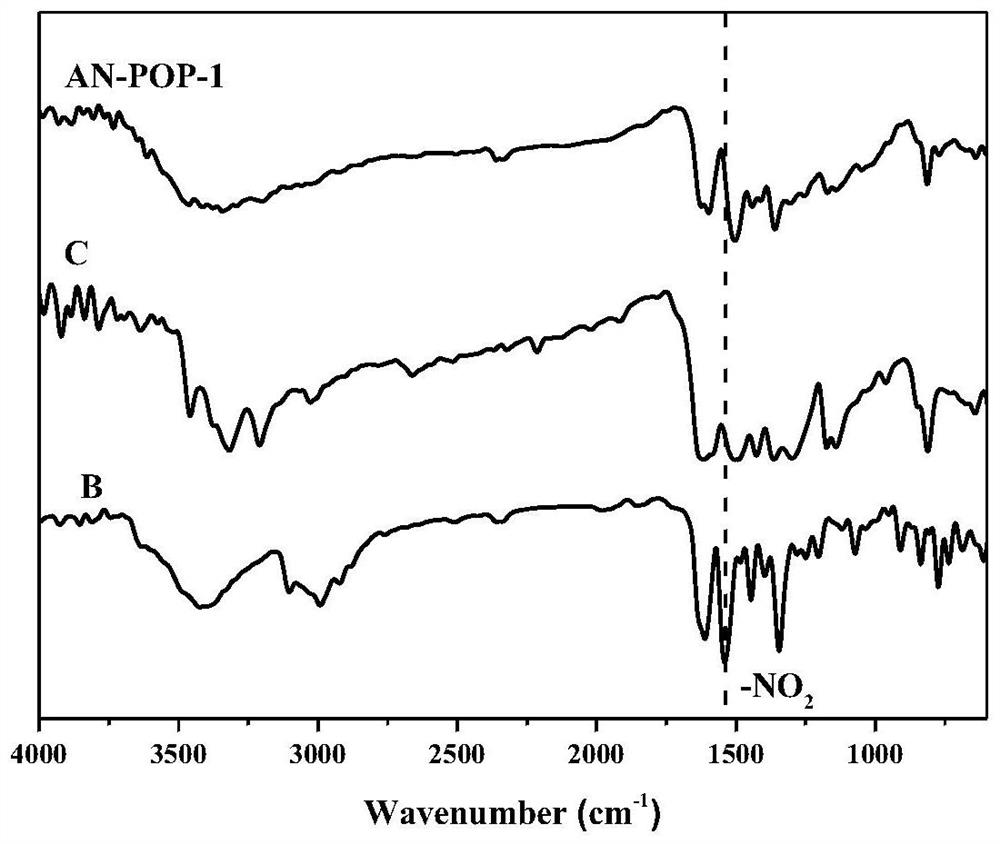
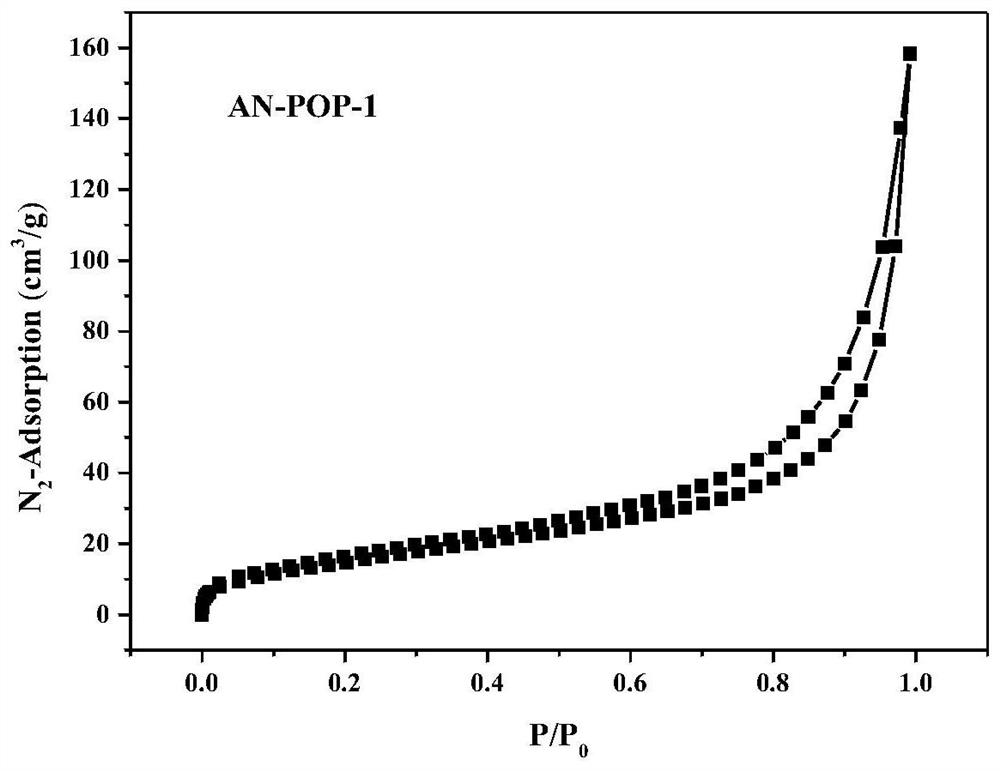
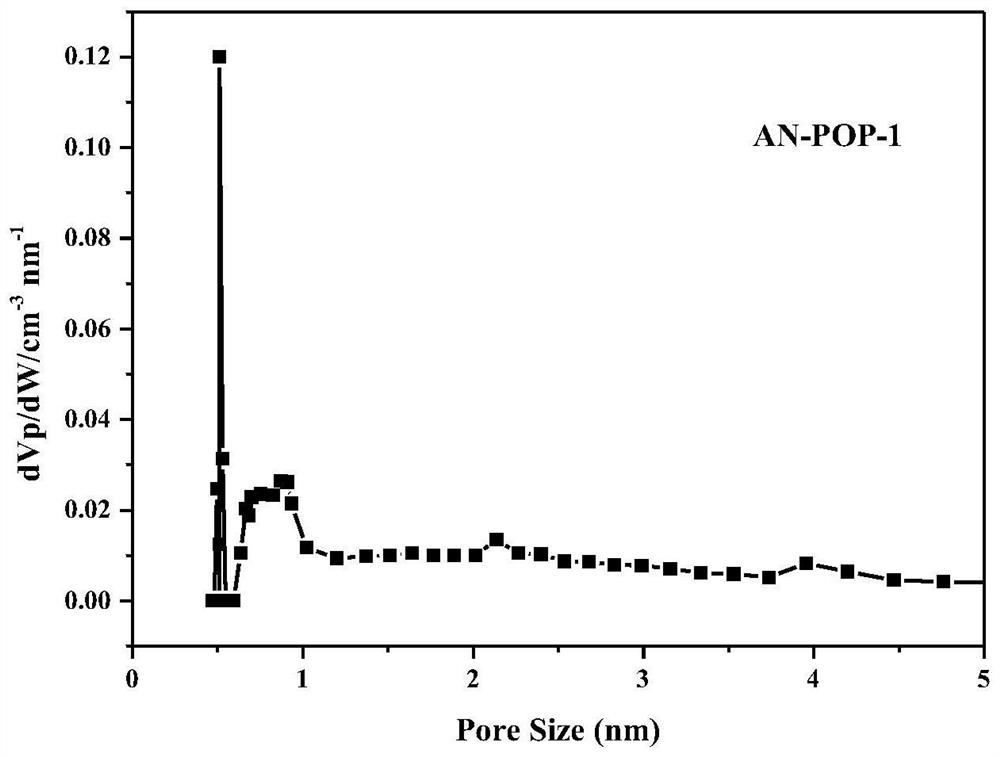
Preparation and catalytic application of donor-acceptor type ionic porous polymer
A technology of porous polymers and donors, applied in the direction of imino compound preparation, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc., to achieve the effect of mild reaction conditions, stable structure and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of preparation method of donor-acceptor type ionic porous polymer iPOPs (AN-POP-1) containing anthracycline, the steps are as follows:
[0049] (1) Preparation of compound A: 9,10-bis(3-pyridyl)anthracene;
[0050]Under argon protection, 9,10-dibromoanthracene (672mg, 2mmol), 4-pyridineboronic acid (615mg, 5mmol), tetrakis (triphenylphosphine) palladium (231mg, 0.2mmol) and potassium carbonate (2.72g , 20mmol) into a 250mL Schlenk bottle. Then 80 mL of DMF solution and 10 mL of water were added successively, and the reaction mixture was placed in an oil bath at 90° C. and stirred at constant temperature for 12 hours. After cooling to room temperature, the solvent was removed in vacuo and the residue was redissolved in a mixture of chloroform and water. The two phases were separated and the organic layer was washed twice with water. Finally, hydrochloric acid (1M) was added to the collected aqueous phase until pH 1 was reached. During acidification, some resid...
Embodiment 2
[0064] The test method for the amine oxide coupling reaction catalyzed by the anthracycline-containing donor-acceptor ionic porous polymer iPOPs (AN-POP-1) prepared in Example 1 is as follows:
[0065] Under an oxygen atmosphere, AN-POP-1 (2.5-5 μmol, 0.5-1mol%) and 4-methylbenzylamine (0.5mmol) prepared in Example 1 were added to a quartz tube, and then 10 μL of dibromomethane was added, and then Add MeCN (5 mL) to disperse the benzylamine evenly, irradiate with a 26W blue LED light at room temperature, and stir. by TLC and 1 The progress of the reaction was monitored by H NMR, and the reaction was stopped after the disappearance of the starting material. The catalyst was then removed by centrifugation, filtration, and the filtrate was concentrated and evaporated to obtain the crude product. Crude product using 1 HNMR analysis, the ratio of the internal standard and the integrated peak of the product was used to calculate the conversion. Characterization data: 1 H NMR (5...
Embodiment 3
[0069] The test method for the amine oxide coupling reaction catalyzed by the anthracycline-containing donor-acceptor ionic porous polymer iPOPs (AN-POP-1) prepared in Example 1 is as follows:
[0070] Under an oxygen atmosphere, AN-POP-1 (2.5-5 μmol, 0.5-1mol%) and 4-methoxybenzylamine (0.5mmol) prepared in Example 1 were added to a quartz tube, and then 10 μL of dibromomethane was added, Then MeCN (5 mL) was added to disperse the benzylamine evenly, irradiated with a 26W blue LED light at room temperature, and stirred. by TLC and 1 The progress of the reaction was monitored by H NMR, and the reaction was stopped after the disappearance of the starting material. The catalyst was then removed by centrifugation, filtration, and the filtrate was concentrated and evaporated to obtain the crude product. Crude product using 1 HNMR analysis, the ratio of the internal standard and the integrated peak of the product was used to calculate the conversion. Characterization data: 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com