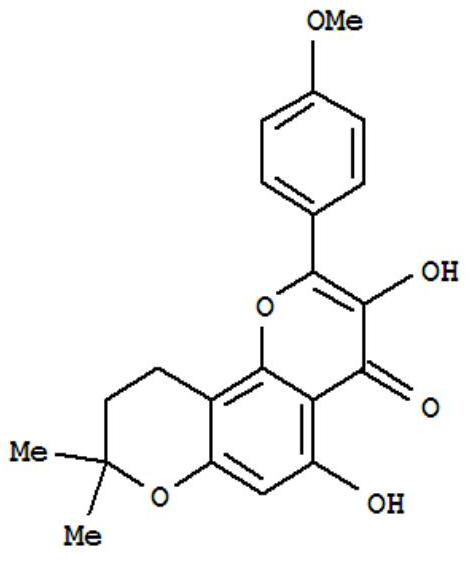
Anhydroicaritin pharmaceutical composition and preparation method thereof
The technology of icariin and composition is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Low bioavailability and other problems, to achieve the effect of increasing dissolution and stability, continuous operation reproducibility, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of solid dispersion
[0032] Party:
[0033]
[0034] Preparation Process:
[0035] (1) Mix the icariin, PEG6000 and triethyl citrate in the prescription amount to make a physical mixture A;
[0036] (2) mix the copovidone of formula quantity and physical mixture A uniformly, make physical mixture B;
[0037] (3) Set the extrusion temperature of the twin-screw hot-melt extruder to 120°C. When the preset temperature is reached, add the physical mixture B in step (2) into the extruder for melting-dissolving- Mix, start the screw after the melting is complete, and extrude strips through the screw.
[0038] (2) Preparation of icarigenin tablet
[0039] Party:
[0040]
[0041] Preparation Process:
[0042] Weigh the icariin solid dispersion prepared in the above (1) and mix with lactose monohydrate, microcrystalline cellulose, sodium carboxymethyl starch and magnesium stearate, and directly compress the powder to obtain Icarigenin Tablets.
Embodiment 2
[0044] (1) Preparation of solid dispersion
[0045] Party:
[0046]
[0047] Preparation Process:
[0048] (1) Mix the icariin, PEG4000 and triethyl citrate in the prescription amount to make a physical mixture A;
[0049] (2) mix the copovidone of formula quantity and physical mixture A uniformly, make physical mixture B;
[0050] (3) Set the extrusion temperature of the twin-screw hot-melt extruder to 100°C. When the preset temperature is reached, add the physical mixture B in step (2) into the extruder for melting-dissolving- Mix, start the screw after the melting is complete, and extrude strips through the screw.
[0051] (2) Preparation of Icarigenin Capsules
[0052] Party:
[0053]
[0054] Preparation Process:
[0055] Weigh the icariin solid dispersion prepared in the above (1) of the recipe, mix with lactose monohydrate, sodium carboxymethyl starch and talcum powder, and mix the powder into capsules to obtain icarigenin capsules .
Embodiment 3
[0057] (1) Preparation of solid dispersion
[0058] Party:
[0059]
[0060] Preparation Process:
[0061](1) Mix the icariin, PEG5000 and triethyl citrate in the prescription amount to make a physical mixture A;
[0062] (2) mix the copovidone of formula quantity and physical mixture A uniformly, make physical mixture B;
[0063] (3) Set the extrusion temperature of the twin-screw hot-melt extruder to 110°C. When the preset temperature is reached, add the physical mixture B in step (2) into the extruder for melting-dissolving- Mix, start the screw after the melting is complete, and extrude strips through the screw.
[0064] (2) Preparation of icarigenin tablet
[0065] Party:
[0066]
[0067] Preparation Process:
[0068] Weigh the icariin solid dispersion prepared in the above (1) of the recipe, mix with microcrystalline cellulose, croscarmellose sodium and micropowder silica gel, and directly compress the powder to obtain Epimedium Aglycone Tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com