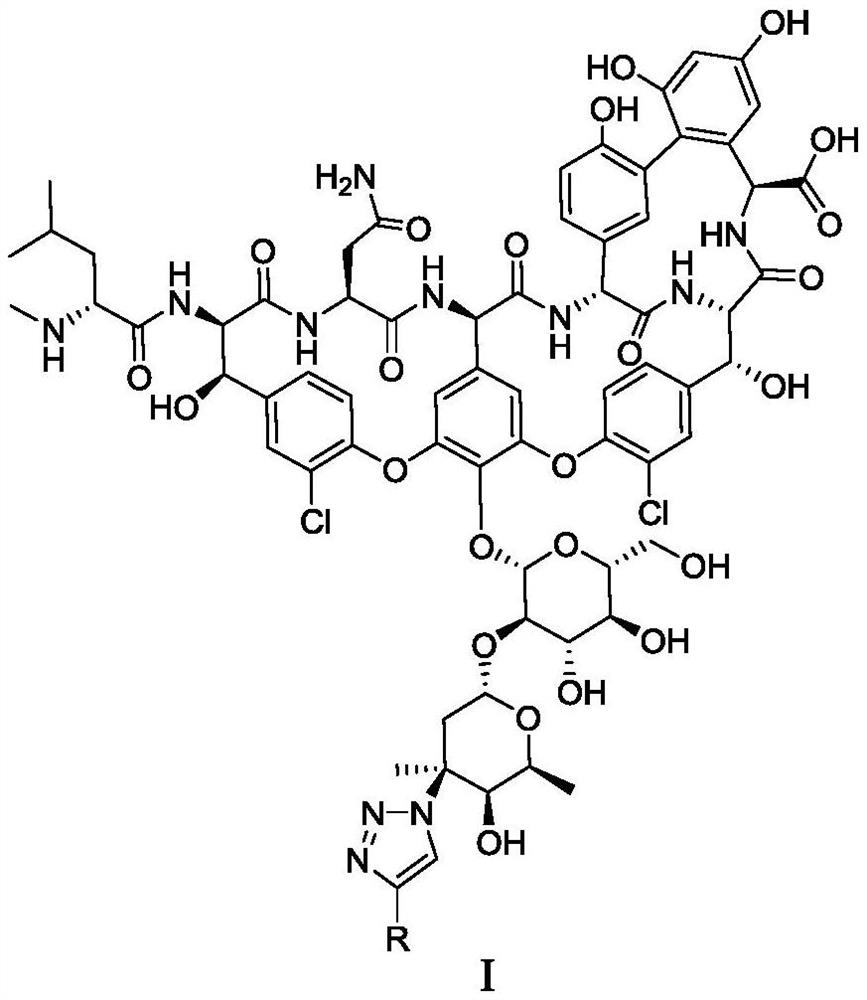
Vancomycin derivative as well as intermediate, preparation method, composition and application thereof
A compound and solvate technology, applied in the field of vancomycin derivatives, can solve the problem of single types of vancomycin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
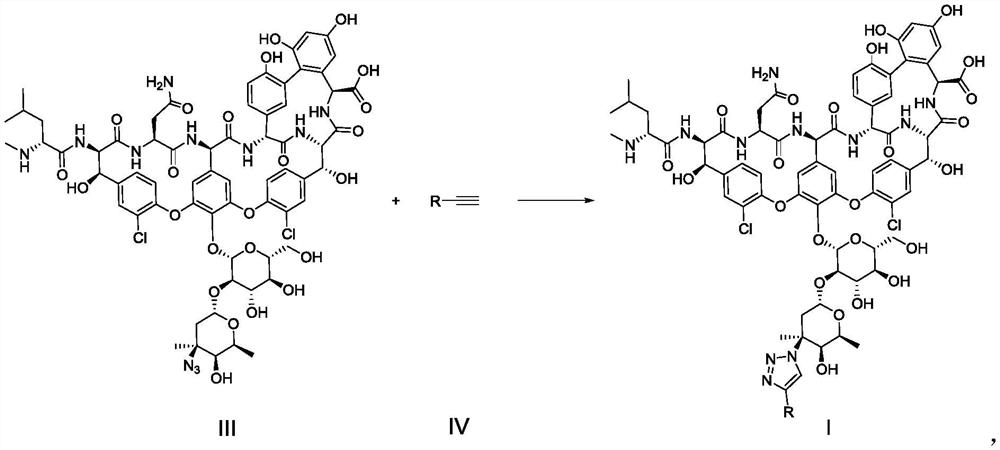
[0177] The synthesis of embodiment 1 vancomycin azide compound
[0178]
[0179]In a 20ml glass bottle, vancomycin hydrochloride (compound 3, 742mg, 0.5mmol; 90% purity) was dissolved in dimethyl sulfoxide (5ml), followed by aqueous potassium bicarbonate (3M, 0.67ml) and fluorosulfonyl Azide solution (500mM, 1.5ml, containing 0.75mmol fluorosulfonyl azide, solvent is methyl tert-butyl ether). The reaction solution was stirred at room temperature for 90 minutes, during which it was detected by LC-MS. After the reaction was completed, the reaction solution was washed with n-hexane (8ml×3). The washed reaction solution was slowly added to vigorously stirred acetone (125 ml) at room temperature. The precipitated insoluble pink solid (992 mg) was isolated by suction filtration, left in air at room temperature for 20 minutes to dry and lightly crushed into powder using a mortar. Distilled water (4 ml) was added to the powder, stirred at room temperature for 20 minutes, and the...
Embodiment 2
[0182]
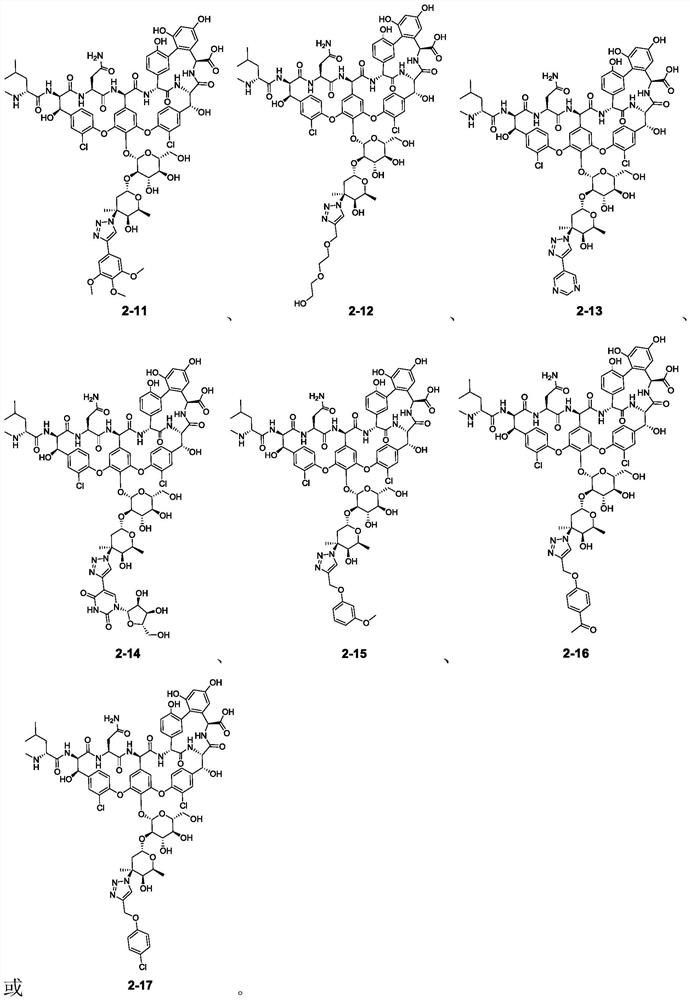
[0183] Add dimethyl sulfoxide (18 μL), water (7 μL), vancomycin-derived azide compound (compound 1, 50 mM solution, solvent is dimethyl sulfoxide, 10 μL, containing 0.5 μmol compound to a 1.5 ml reaction tube in sequence 1), the corresponding terminal alkyne substrate (compound 4, 100mM, the solvent is dimethyl sulfoxide, 5 μL, containing 0.5 μmol terminal alkyne), copper sulfate and tris[(1-(3-hydroxypropyl)-1H- Mixed aqueous solution of 1,2,3-triazol-4-yl)methyl]amine (THPTA) (5 μL, containing 0.1 μmol copper sulfate and 0.2 μmol THPTA) and sodium ascorbate aqueous solution (500 mM, 5 μL, containing 2.5 μmol sodium ascorbate) , reacted on a shaking table at room temperature for 12 hours. After the reaction, the reaction solution was directly diluted to obtain the corresponding vancomycin-derived 1,2,3-triazole compound (ie, compound 2-1~2-4, 2-7 ~2-17) solution (as shown in Table 1 and Table 2), used for biological activity test.
[0184] Table 1
[0185]
...
Embodiment 3
[0189] Embodiment 3 biological activity test
[0190] Dilute MRSA USA300 bacteria with MH medium to a concentration of 1*10 5 CFU / mL, 120 μL of bacterial suspension was added to each well of a 96-well plate, and then different concentrations of compounds were added (the compound concentration was diluted downward with a 2-fold gradient starting from 100 μM), and each compound was set up in three replicates at different concentrations. hole. After culturing in a 37°C incubator for 18 hours, the absorbance value was measured with a microplate reader. The concentration at which bacteria do not proliferate significantly is the minimum inhibitory concentration (MIC).
[0191] The MIC data of compounds 1, 2-1~2-4, 2-7~2-17 obtained in Examples 1 and 2 are as follows, and the blank control item is prepared according to Example 2 but does not contain compound 1 and terminal Reaction solution of alkyne substrate. The test results are shown in Table 3.
[0192] The minimum inhibito...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com