Application of macromolecular protein in anti-RNA virus disinfectant
A technology of RNA viruses and macromolecules, applied in the field of anti-RNA virus disinfectants of macromolecular proteins, can solve the problems of few studies on physical and chemical stability, protein instability, variability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Disclosed is a disinfectant for RNA viruses that acts on the outside or body surface, and the active ingredient of the disinfectant is recombinant RNase2 and / or RNase3.
[0046] Recombinant RNase2 and recombinant RNase3 are recombinant human-derived proteins, which can be expressed and purified by recombinant DNA from E.coli (Escherichia coli), Sf9 insect cells or human 293 cells, and the purity of the protein is greater than 90%, and the concentration is 1~ 200 μM. The preparation of recombinant RNase2 and recombinant RNase3 is a prior art, and will not be repeated here.
[0047] Water is used as a solvent, and the concentration of recombinant RNase2 and / or RNase3 is 0.01-5000nM.
[0048] In order to maintain long-term activity and improve the use effect of the disinfectant, protein stabilizers, phosphate buffers, and preservatives can also be added to the disinfectant. The protein stabilizer includes one or more of antiseptic and antibacterial protein stabilizers, p...
Embodiment 2
[0052] Stability verification of embodiment 2 disinfectant
[0053] When the anti-RNA virus disinfectant in Example 1 is applied to the body surface or in vitro, it should first be ensured that the recombinant RNase2 and recombinant RNase3 have sufficient stability, can be properly stored for a long time, and can not be easily destroyed by the natural environment in various environments. It can be rapidly destroyed, degraded and inactivated by conditions and conditions, and can be mixed with other auxiliary ingredients.
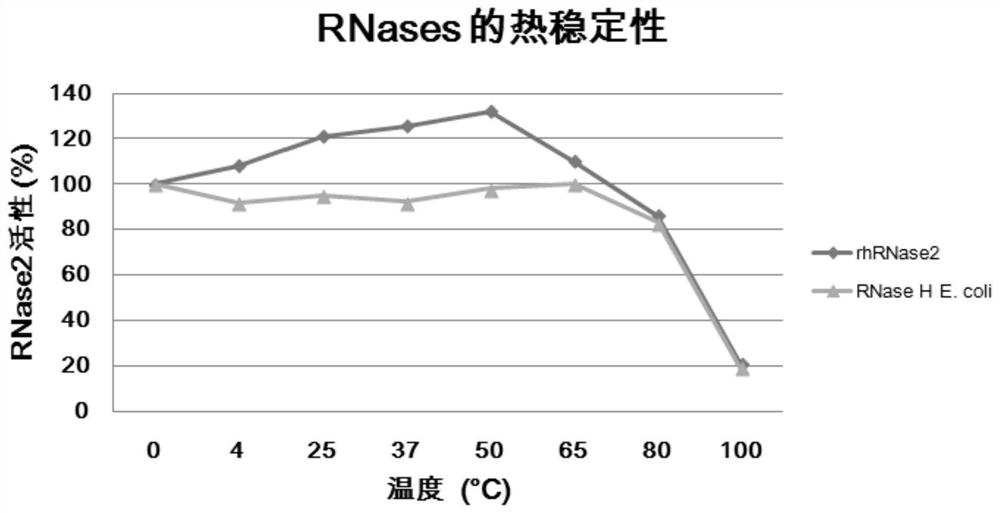
[0054] (1) Thermal stability of recombinant RNase2 and recombinant RNase3
[0055] Under various temperature conditions, the ribonuclease activity of RNase2 was tested. Ribonuclease assay: catalytic reaction Add 40mg of yeast tRNA as substrate to 0.8mL reaction solution (containing 40mM sodium phosphate, pH 7.0 and 10mL rhRNase2, or rRNaseH, or buffer control). Reactions were terminated by adding 40 nM lanthanum nitrate in ice-cold 3% perchloric acid at giv...
Embodiment 3
[0069] The anti-RNA virus ability of embodiment 3 disinfectant
[0070] Treat the same amount of respiratory syncytial virus and parainfluenza virus (PIV) with different doses of recombinant RNase2, then use the treated virus to infect the cultured cells, and detect the number of active viruses. The results are shown in the table below (all experiments were repeated 3 times, each 2 times, the result is the mean, ± standard deviation):
[0071]
[0072] It can be seen from the above table that the number of active viruses decreases with the increase of the amount of RNase2, which proves that RNase2 has inhibitory and destructive effects on both viruses, but the sensitivity of the viruses to RNase2 is different. Its inferred reason is: respiratory syncytial virus and parainfluenza virus all have Matrix Proteins (such as Figure 8 shown), is a key protein in the structure of the virus, and plays an important role in assembling the virus, protecting the viral envelope and geno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com