Method for synthesizing progesterone
A progesterone and compound technology, applied in the field of progesterone synthesis, can solve the problems of high cost and low yield, and achieve the effect of cheap raw materials, simple reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
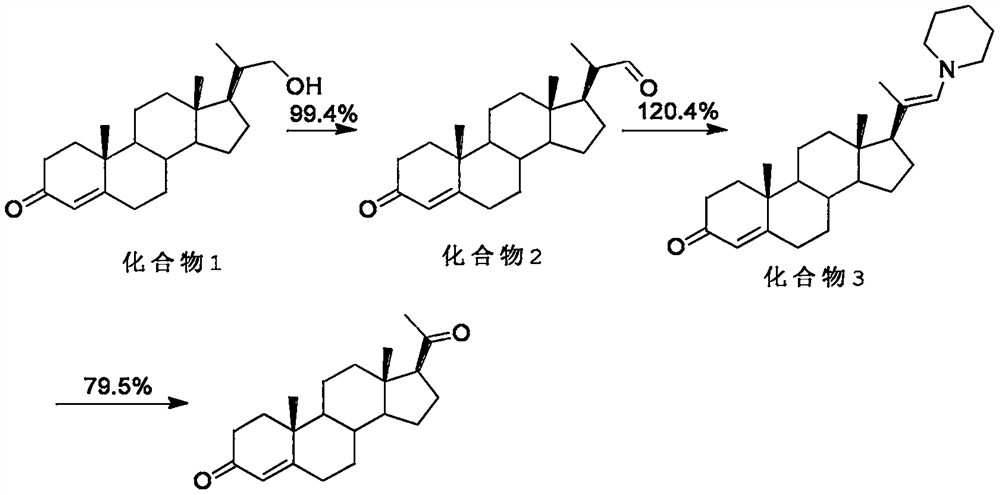
[0021] see figure 1 , the present invention provides a technical scheme: a method for progesterone synthesis, comprising the following steps:
[0022] S1: Synthesis of compound 2, 100g of compound 1 (derivative of 4AD), tetramethylpiperidine oxide in a 2L single-necked bottle, add 3V dichloromethane to dissolve, stir, add sodium bromide, sodium bicarbonate aqueous solution, and then Add 10V sodium hypochlorite solution, stir at 25°C for 16-20h, TLC detects that the reaction of the raw materials is complete, separate the liquid, extract twice with 300mL dichloromethane, combine the organic phase, wash once with water, concentrate to dryness, entrain with methanol twice, then add 10V methanol, concentrated to 3-5V volume, under stirring, 7V water was added dropwise, filtered, and dried at 50°C to obtain 90g of compound 2, HPLC > 98;
[0023] S2: Synthesis of compound 3: under nitrogen protection: add 1-(1-piperidinyl)cyclohexene (self-made) to 2V acetonitrile, heat up to 65-68°...
Embodiment 2
[0027] see figure 1 , the present invention provides a technical scheme: a method for progesterone synthesis, comprising the following steps:
[0028] S1: Synthesis of compound 2, 100g of compound 1 (derivative of 4AD), tetramethylpiperidine oxide in a 2L single-necked bottle, add 3V chloroform to dissolve, stir, add sodium bromide, sodium bicarbonate aqueous solution, and then Add 10V sodium hypochlorite solution, stir at 25°C for 16-20h, TLC detects that the reaction of the raw materials is complete, separate the liquid, extract twice with 300mL chloroform, combine the organic phase, wash once with water, concentrate to dryness, entrain with methanol twice, then add 10V methanol, concentrated to 3-5V volume, under stirring, 7V water was added dropwise, filtered, and dried at 50°C to obtain 90g of compound 2, HPLC > 98;
[0029]S2: Synthesis of compound 3: under nitrogen protection: add 1-(1-piperidinyl)cyclohexene (self-made) to 2V acetonitrile, heat up to 65-68°C to dissol...
Embodiment 3
[0033] see figure 1 , the present invention provides a technical scheme: a method for progesterone synthesis, comprising the following steps:
[0034] S1: Synthesis of compound 2, 100g of compound 1 (derivative of 4AD), tetramethylpiperidine oxide in a 2L single-necked bottle, add 3V dichloromethane to dissolve, stir, add sodium bromide, sodium bicarbonate aqueous solution, and then Add 10V sodium hypochlorite solution, stir at 25°C for 16-20h, TLC detects that the reaction of the raw materials is complete, separate the liquid, extract twice with 300mL dichloromethane, combine the organic phase, wash once with water, concentrate to dryness, entrain with methanol twice, then add 10V methanol, concentrated to 3-5V volume, under stirring, 7V water was added dropwise, filtered, and dried at 50°C to obtain 90g of compound 2, HPLC>98:
[0035] S2: Synthesis of compound 3: under nitrogen protection: add 1-(1-piperidinyl)cyclohexene (self-made) to 2V acetonitrile, heat up to 65-68°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com