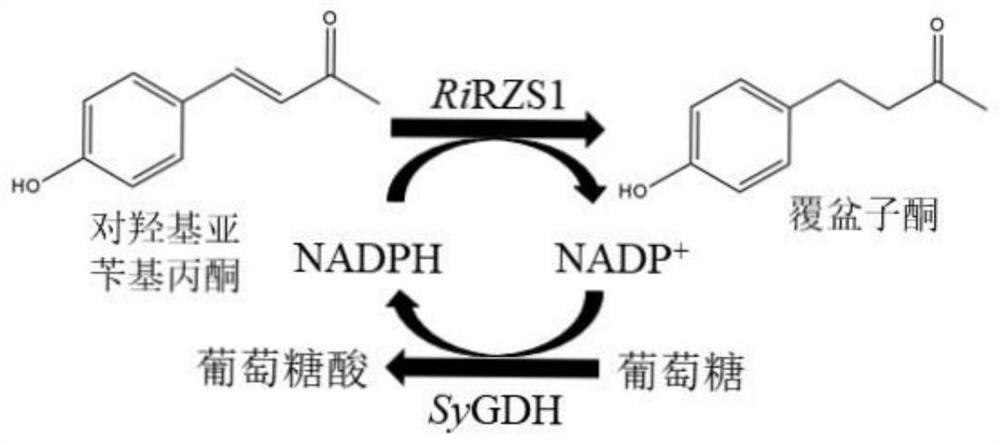
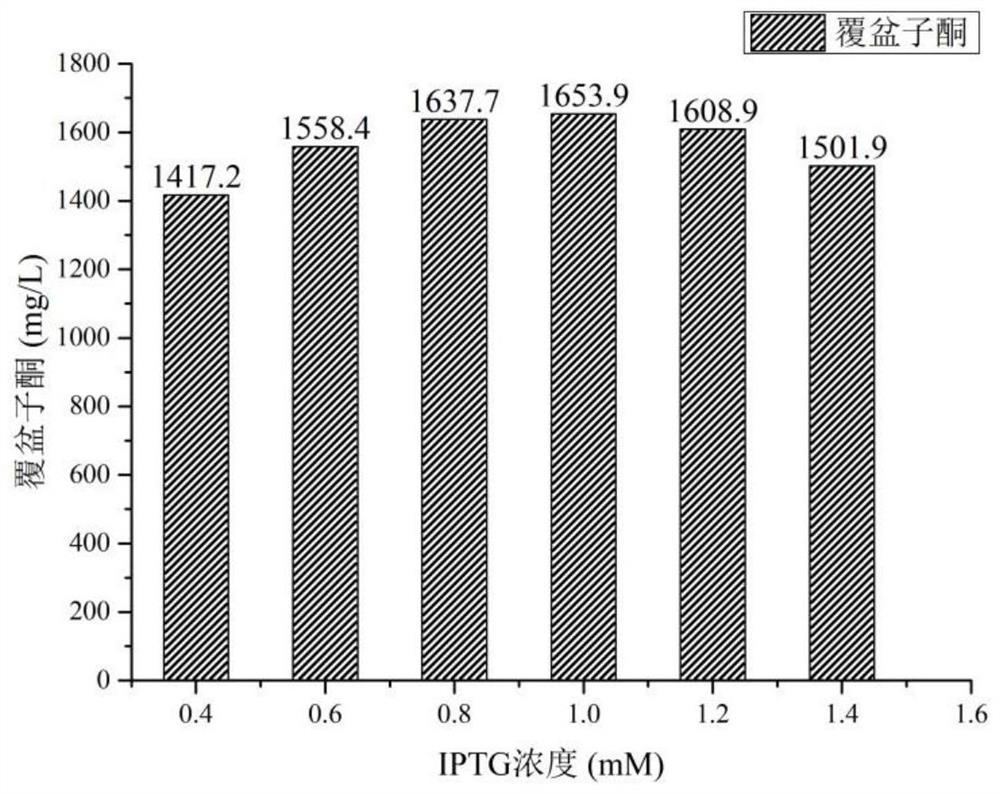
A method for preparing raspberry ketone by whole cell transformation
A technology of whole cell transformation and raspberry ketone, which is applied in the field of genetic engineering, can solve problems such as complex preparation methods, long fermentation time, and equipment corrosion, and achieve the effects of environmental protection, high conversion efficiency, and low energy consumption in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Construction of the recombinant plasmid containing glucose dehydrogenase gene sygdh
[0059] Specific steps are as follows:
[0060] Using the codon-optimized nucleotide sequence such as the glucose dehydrogenase gene sygdh shown in SEQ ID NO.2 as a template, PCR amplification was performed with sygdh-F and sygdh-R as primers; the PCR reaction conditions were: 90 Pre-denaturation at -95°C for 3 to 5 minutes, denaturation at 94°C for 30 to 45 seconds, annealing at 55°C for 30 to 45 seconds, extension at 70 to 72°C for 1 minute, 30 cycles, and full extension at 70 to 72°C for 5 to 10 minutes.
[0061] After the PCR reaction, the PCR product was detected by agarose gel electrophoresis, and the glucose dehydrogenase gene sygdh was recovered by cutting the gel. The primers are:
[0062] sygdh-F: GGATCCATGACCGAACAGAAAG (SEQ ID NO. 3)
[0063] sygdh-R: AAGCTTGCGGCCGCTTACTGC (SEQ ID NO. 4)
[0064] Ligate the glucose dehydrogenase gene sygdh obtained in the ab...
Embodiment 2
[0065] Example 2: Construction of recombinant plasmids containing glucose dehydrogenase gene sygdh and benzylacetone reductase gene rirzs1
[0066] Specific steps are as follows:
[0067] The benzylacetone reductase gene rirzs1 whose codon-optimized nucleotide sequence is shown in SEQ ID NO.1 was used as a template, and rirzs1-F and rirzs1-R were used as primers for PCR amplification; the PCR reaction conditions were: Pre-denaturation at 90-95°C for 3-5min, denaturation at 94°C for 30-45sec, annealing at 55°C for 30-45sec, extension at 70-72°C for 1min, 30 cycles, full extension at 70-72°C for 5-10min.
[0068] After the PCR reaction, the PCR product was detected by agarose gel electrophoresis, and the benzylacetone reductase gene rirzs1 was recovered by cutting the gel. The primers are:
[0069] rirzs1-F: AGATCTATGGCCAGCGGC (SEQ ID NO. 5)
[0070] rirzs1-R: GGTACCTTATTCACGGCTAACCA (SEQ ID NO. 6)
[0071] The benzyl acetone reductase gene rirzs1 obtained in the above steps...
Embodiment 3
[0072] Example 3: Construction of recombinant Escherichia coli co-expressing benzyl acetone reductase and glucose dehydrogenase
[0073] Specific steps are as follows:
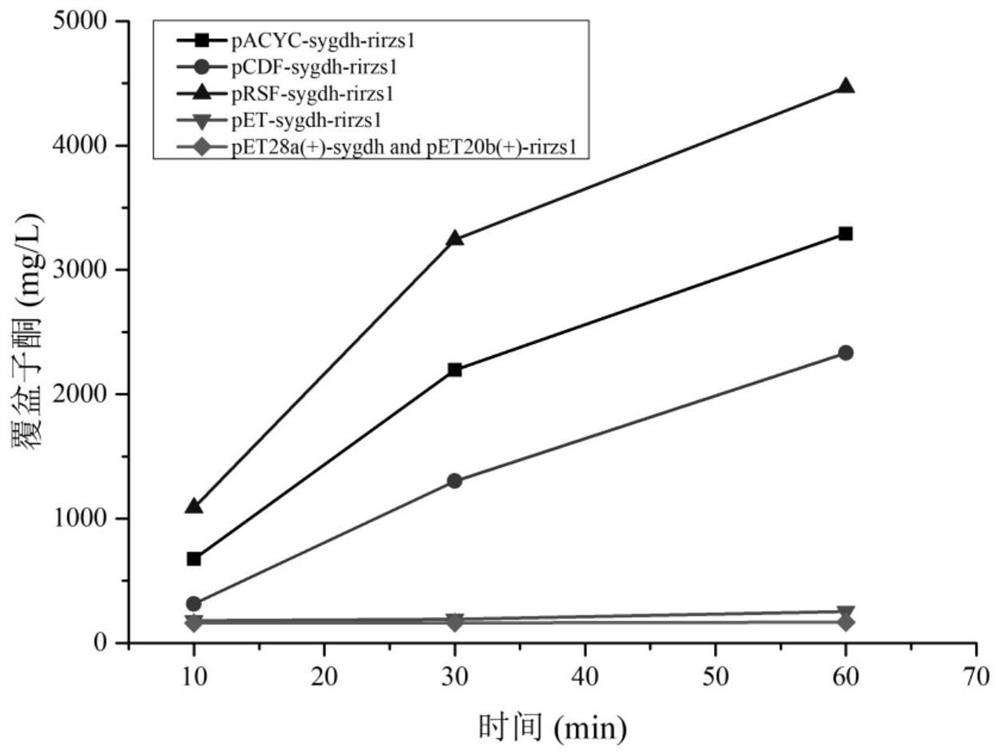
[0074] (1) The recombinant plasmid pET-sygdh-rirzs1 constructed in Example 2, pACYC-sygdh-rirzs1, pCDF-sygdh-rirzs1 and pRSF-sygdh-rirzs1 were transformed into Escherichia coli BL21 (DE3) competent cells respectively to obtain Single plasmid recombinant engineering strains BL21 / pET-sygdh-rirzs1, BL21 / pACYC-sygdh-rirzs1, BL21 / pCDF-sygdh-rirzs1 and BL21 / pRSF-sygdh-rirzs1.
[0075] (2) Construction of recombinant engineering strains containing dual plasmid expression vectors pET20b(+)-rirzs1 and pET28a(+)-sygdh
[0076] With codon optimized nucleotide sequence such as glucose dehydrogenase gene sygdh shown in SEQ ID NO.2 and codon optimized nucleotide sequence such as the benzyl acetone reduction shown in SEQ ID NO.1 The enzyme gene rirzs1 is used as a template, and sygdh-F-Nco I, sygdh-R-Xho I, rirzs1-F-Nde I,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com