Infant formula milk powder containing gastrointestinal regulation peptide and preparation method thereof
A technology for infant formula and milk powder, which is applied in the direction of milk preparations, dairy products, applications, etc., and can solve problems such as bitter taste, low lactose content, and infant malnutrition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]An infant formula containing gastrointestinal regulatory peptides, including the following components in weight ratio:
[0055]Infant formula milk powder 100g
[0056]Motilin 193.93ng
[0057]14 peptide somatostatin 310.29ng;
[0058]The chemical formula of motilin is C120H188N34O35S, the molecular weight is 2699.05, and the concentration is 250ng / L;
[0059]The chemical formula of the 14 peptide somatostatin is C76H104N18O19S2, The molecular weight is 1638, and the concentration is 400ng / L.
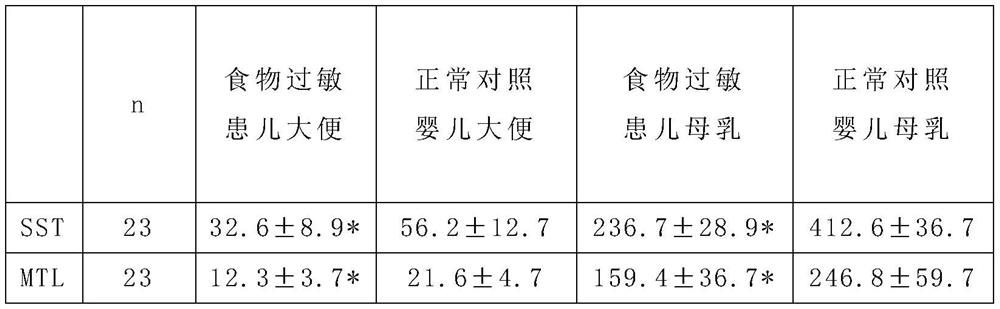
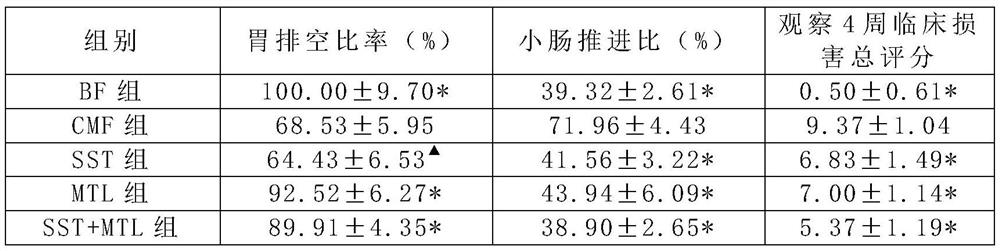
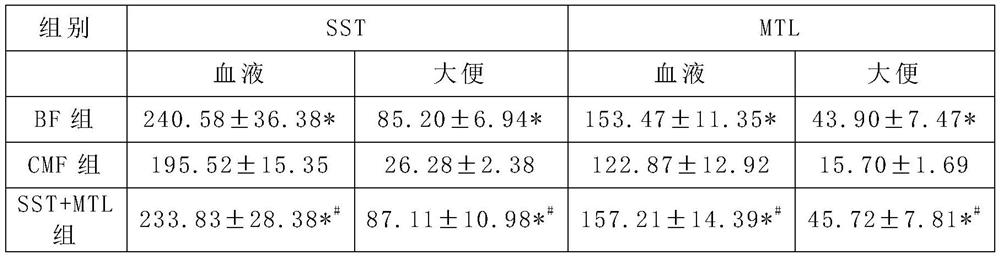
[0060]Somatostatin SST can significantly reduce the allergenicity of ordinary milk powder, significantly reduce the allergic digestive tract injury induced by milk protein, and reduce the risk of milk allergy; Motilin MTL can significantly improve the gastrointestinal tolerance of ordinary milk powder , And can well improve baby’s appetite and gastrointestinal motility; the simultaneous addition of somatostatin SST and motilin MTL has the effect of offsetting mutual side effects and synergistically regula...
Embodiment 2
[0062]An infant formula containing gastrointestinal regulatory peptides, including the following components in weight ratio:
[0063]Infant formula milk powder 100g
[0064]Motilin 193.93ng
[0065]14 peptide somatostatin 310.29ng;
[0066]The chemical formula of motilin is C120H188N34O35S, the molecular weight is 2699.05, and the concentration is 250ng / L;
[0067]The chemical formula of the 14 peptide somatostatin is C76H104N18O19S2, The molecular weight is 1638, and the concentration is 400ng / L.
[0068]The weight ratio components of the infant formula milk powder are 95.16 g of whole milk powder, 2.80 g of linoleic acid, 375 g of α-linoleic acid, 396 ug of vitamin A, 7.00 ug of vitamin D, and 6.00 mg of vitamin E per 100 g. Vitamin K134.00ug, vitamin B1 380.00ug, vitamin B2 700ug, vitamin B6 280ug, vitamin B12 0.9ug, niacin 3500ug, folic acid 70.0ug, pantothenic acid 2600ug, vitamin C 70mg, biotin 11.4ug, sodium 150mg, potassium 531mg, copper 290ug, magnesium 48mg, iron 5.6mg, zinc 3.30mg, mangane...
Embodiment 3
[0072]A method for preparing infant formula milk powder containing gastrointestinal regulatory peptides includes the following steps:
[0073]a. Preparation of raw milk: raw milk is purified and filtered. Use skim milk powder, whey protein powder and mixed vegetable oil to adjust the ingredients to a standardized content. After mixing, filter again and homogenize, pre-cook at 80°C for 20 seconds to sterilize, and cool to 4℃ standby;
[0074]b. Premixes with nutritional fortification ingredients: including water-soluble vitamin premixes, fat-soluble vitamin premixes, mineral and trace element premixes, unsaturated fatty acid premixes, nucleotide premixtures and amino acid premixtures ;
[0075]c. Configure active gastrointestinal regulatory peptide premix: each active gastrointestinal regulatory peptide premix includes 25ng of motilin and 40ng of 14 peptide somatostatin;
[0076]d. Mixing and homogenization: mix the emulsion and pre-mixed ingredients in the following proportions, 100ml of standa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com