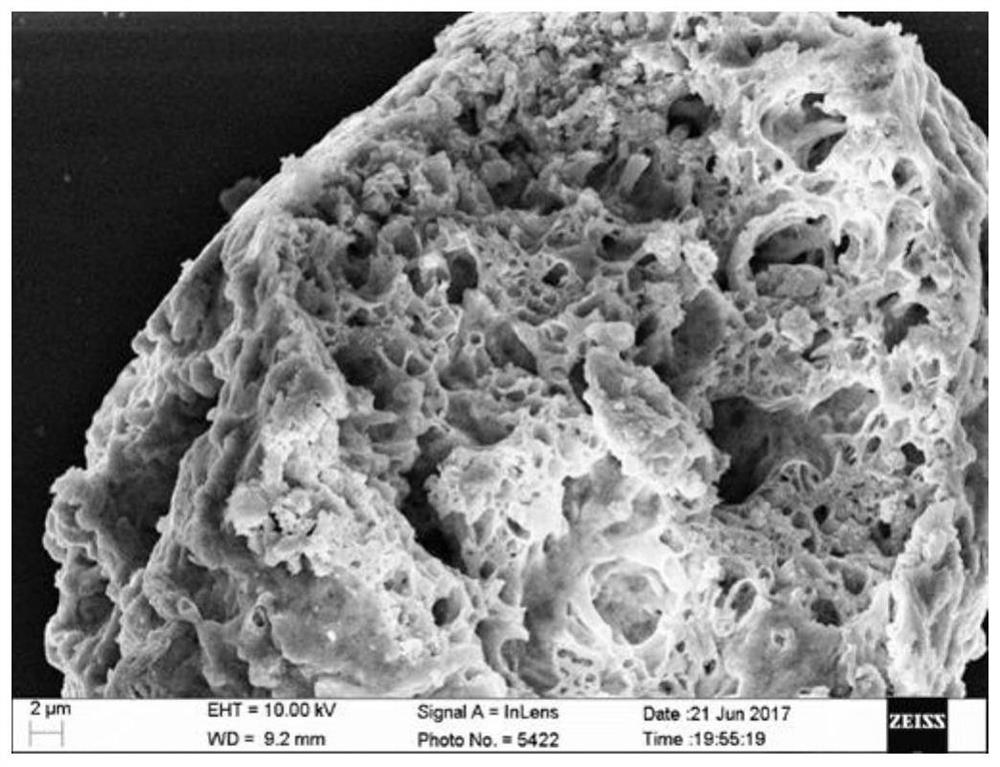
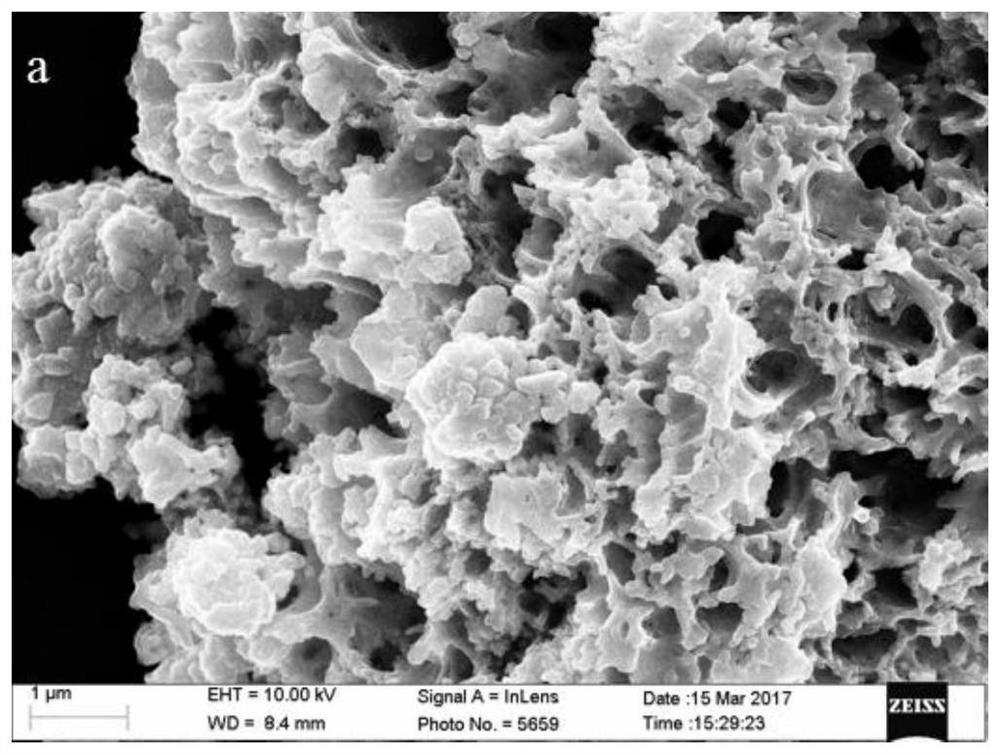
Preparation method of bimetallic co-doped cerium dioxide catalyst with porous structure
A technology of ceria and porous structure, applied in the field of double metal co-doped ceria, the catalyst preparation field, can solve the problems of poor stability, achieve good stability, enrich oxygen vacancies and defects, and improve catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Ce 0.7 mn 0.3 Rh 0.02 o 2-x preparation of
[0020] S1) Weigh 75mmol (9.454g) of oxalic acid dihydrate and pour it into a 200mL beaker, add 75mL of deionized water, and stir at a speed of 800rpm for 10 minutes to form a clear aqueous solution of oxalic acid;
[0021] S2) take by weighing 7mmol (3.040g) cerium nitrate hexahydrate and 3mmol (0.7352g) manganese acetate tetrahydrate and 0.2mmol (0.0527g) rhodium chloride trihydrate in a 100mL beaker, add 75mL deionized water, Stir for 10 minutes at a speed of 800rpm to form a clear metal salt solution;
[0022] S3) Pour the metal salt solution into the oxalic acid solution, stir at 800rpm for 6 hours, and then stand at 50°C for 12h;
[0023] S4) After separating the above solution, washing with deionized water, drying at 80° C. for 12 hours;
[0024] S5) The dried sample was heated to 500°C at a heating rate of 2°C / min in air atmosphere and kept for 4 hours to obtain Ce 0.7 mn 0.3 Rh 0.02 o 2-x catalyst...
Embodiment 2
[0025] Example 2: Ce 0.7 Fe 0.3 Rh 0.02 o 2-x preparation of
[0026] S1) Weigh 75 mmol (9.454 g) of oxalic acid dihydrate and pour it into a 200 mL beaker, add 75 mL of deionized water, and stir at a speed of 800 rpm for 20 minutes to form a clear aqueous oxalic acid solution;
[0027] S2) Weigh 7mmol (3.040g) of cerium nitrate hexahydrate and 3mmol (0.8342g) of ferrous sulfate heptahydrate and 0.2mmol (0.0527g) of rhodium chloride trihydrate in a 100mL beaker, add 75mL of deionized water , stirred at a speed of 800rpm for 10 minutes to form a clear metal salt solution;
[0028] S3) Pour the metal salt solution into the oxalic acid solution, stir at 800rpm for 4 hours, and then stand at 60°C for 18h;
[0029] S4) After separating the above solution, washing with deionized water, drying at 100° C. for 24 hours;
[0030] S5) The dried sample was heated to 400°C at a heating rate of 5°C / min in the air atmosphere and kept for 6 hours to obtain Ce 0.7 Fe 0.3 Rh 0.02 o 2-...
Embodiment 3
[0031] Example 3: Ce 0.7 Cr 0.3 PD 0.02 o 2-x preparation of
[0032] S1) Weigh 150mmol oxalic acid (19.100g) and pour it into a 200ml beaker, add 75mL deionized water, and stir at 800rpm for 10 minutes to form a clear oxalic acid aqueous solution;
[0033] S2) Weigh 14mmol (6.0791g) of cerium nitrate hexahydrate and 6mmol (2.4009g) of chromium nitrate nonahydrate and 0.4mmol (0.090g) of palladium acetate in a 100ml beaker, add 75mL of water, and stir at a speed of 800rpm 10 minutes, a clear metal salt solution was formed;
[0034] S3) Pour the metal salt solution into the oxalic acid solution, stir at 800rpm for 4 hours, and then stand at 40°C for 12h;
[0035] S4) After separating the above solution, washing with deionized water, drying at 100° C. for 24 hours;
[0036]S5) The dried sample was heated to 400°C at a heating rate of 4°C / min in the air atmosphere and kept for 6 hours to obtain Ce 0.7 Cr 0.3 PD 0.02 o 2-x catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com