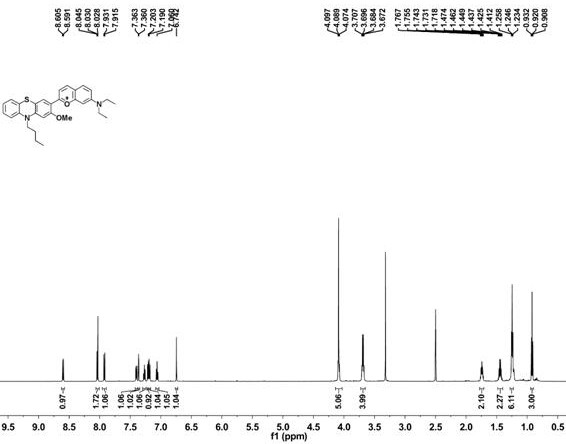
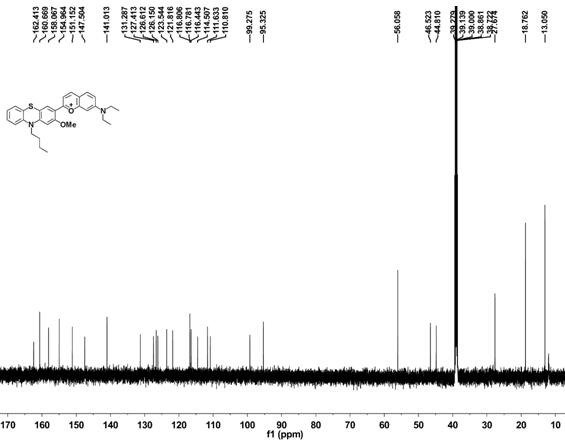
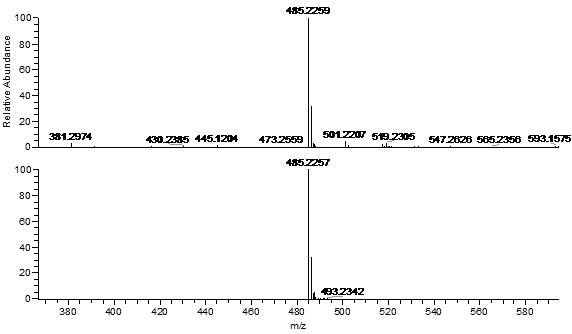
2-(10-buty1-2-methoxy-10H-phenothiazin-3-yl)-7-(diethylamino)chromenylium perchlorate derivative as well as preparation method and application thereof
A technology of phenothiazine derivatives and benzopyrylium, which is applied in the field of chemical synthesis, can solve problems such as high detection limit, poor water solubility, and inability to realize real-time quantitative detection of HClO
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Synthesis of benzopyrylium-phenothiazine derivatives
[0055] (1) Synthesis of 10-n-butyl-2-methoxyphenothiazine
[0056]
[0057] Under argon atmosphere, 2-methoxyphenothiazine (5 mmol, 1.145 g), bromobutane (10 mmol, 1.36 g), sodium hydroxide (10 mmol, 0.4 g) and potassium iodide (0.072 mmol, 12 mg) was added to dimethyl sulfoxide (10 mL), the reaction system was reacted at 95 °C for 6 h, cooled to room temperature, quenched by adding 100 mL of water, followed by extraction with dichloromethane, the organic phase was collected and washed with anhydrous Drying over sodium sulfate, removing the organic solvent under reduced pressure, the resulting sample was separated by column chromatography (petroleum ether as the eluent) to obtain 10-n-butyl-2-methoxyphenothiazine (compound 1) as a colorless oily liquid, producing The rate is 89%.
[0058] (2) Synthesis of 1-(10-n-butyl-2-methoxy-phenothiazin-3-yl)ethanone
[0059]
[0060] Under ice bath, compound...
Embodiment 2
[0067] Example 2 Detection of HClO by Benzopyrylium-Phenothiazine Derivative PTC in Aqueous Phase System
[0068] Add 2.98mL PBS (10 mM, pH = 7.4) buffer solution and 10 μL benzopyrylium-phenothiazine derivative PTC (3 mM) to a series of fluorescence cuvettes successively, then add HClO, ONOO - , HO • , H 2 o 2 , O 2 −• , t -BuOOH,NO, Cys, Hcy, GSH, L-Ser, DL-Met, L-Phe, L-Lys, L-Leu, L-Pro, L-His,S 2- , NO 3 - ,NO 2 - , HSO 3 - , OAc - , SO 4 2- , PO 4 3- , K + , Zn 2+ , Cu 2+ , Na + (30 mM), if the fluorescence intensity of benzopyrylium-phenothiazine derivative PTC in PBS buffer at 627nm is significantly enhanced, indicating that HClO is added; if benzopyrylium-phenothiazine derivative PTC The fluorescence intensity of the PBS buffer solution at 627nm did not change significantly, indicating that it was not HClO that was added.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com