Chitin binding domain and application thereof
A technology of chitin binding domain and identity, applied in the field of enzyme engineering, can solve the problems of increasing the investment of relevant reagents and equipment, increasing the cost of using immobilized enzymes, lack of specificity, etc., and achieving strong chitin specific binding ability. , Simplified immobilization process, easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of a general expression vector with a chitin-binding domain
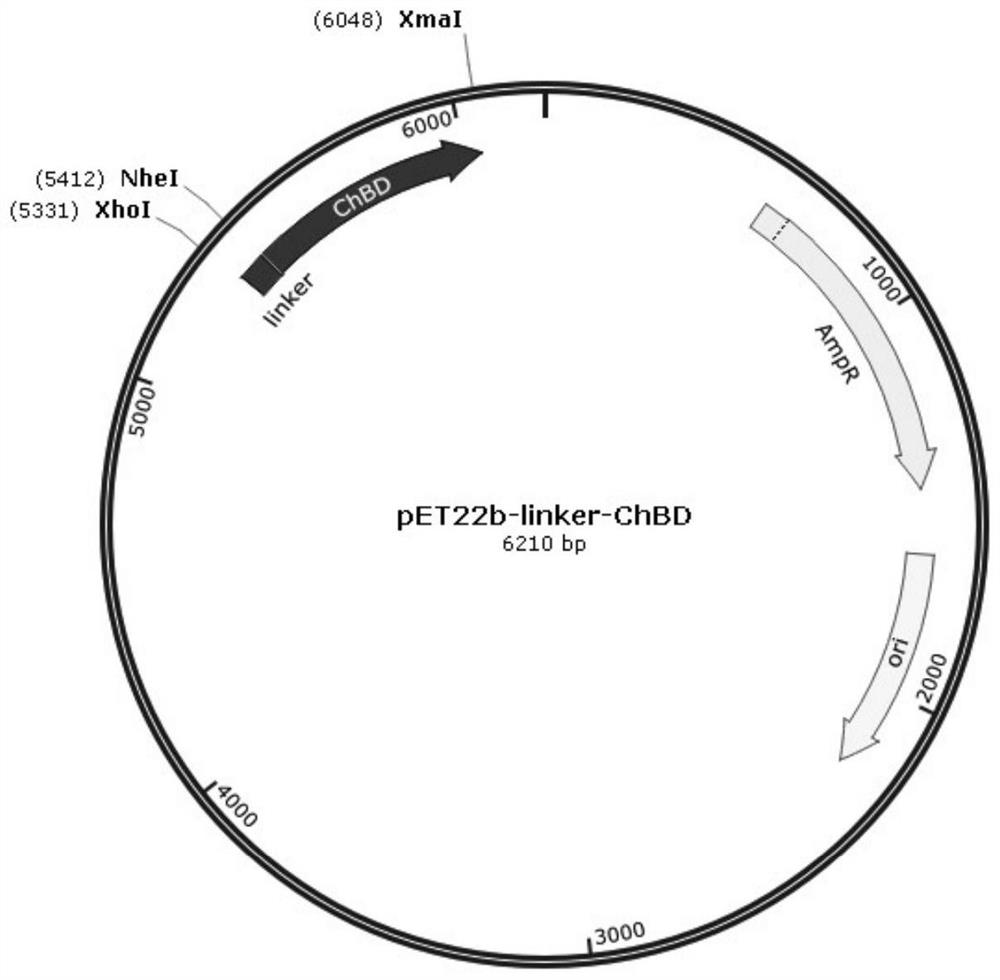
[0052] A flexible linker peptide was selected to link with the chitin-binding domain to construct a universal expression vector. Wherein the amino acid sequence of the chitin-binding domain is shown in SEQ ID No.1, and the nucleotide sequence is shown in SEQ ID No.2; the amino acid sequence of the flexible linker peptide is shown in SEQ ID No.3, and the nucleotide sequence As shown in SEQ ID No.4. The gene connecting the peptide is located at the 5' end of the chitin binding domain gene and inserted into the pET22b vector to obtain the general expression vector pLChBD. The plasmid map of the general expression vector pLChBD is as follows: figure 1 shown. Plasmid pLChBD was maintained in E. coli DH5α.
Embodiment 2
[0053] Example 2: Construction of recombinant β-glucosidase with chitin binding domain
[0054] Using the genome of the acidophilic heat-resistant bacterium Acidothermus cellulolyticus 11B (ATCC 43068) as a template, it was designed according to the gene sequence of its β-glucosidase (the nucleotide sequence is SEQ ID No.5, and the amino acid sequence is SEQ ID No.6). For primers, select NdeI and XhoI restriction sites, and design primers P1 and P2 as follows:
[0055] P1: 5'-CGACTTCATATGATGACACAAATCGAAGAGCG-3'
[0056] P2: 5'-ACCGCTCGAGTCAGGGCGCCGCGATCGTGTTC-3'
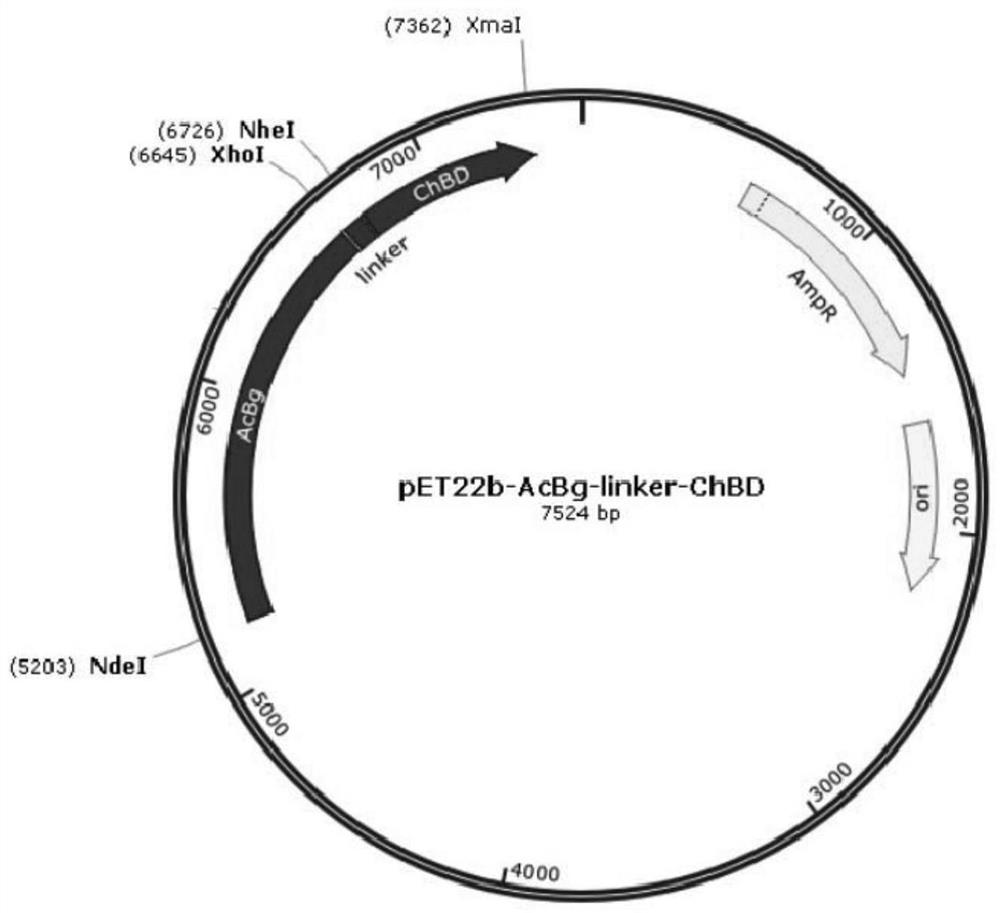
[0057] The PCR amplified fragments with restriction enzymes NdeI and XhoI were also added to the general expression vector pLChBD with restriction enzymes NdeI and XhoI, and double enzyme digestion was carried out at 37°C for 3 hours. The product of the above double digestion was purified with a DNA purification kit. After the two purified products were mixed, T4 DNA ligase from Quanshijin Company was added, and l...
Embodiment 3
[0058] Example 3: Preparation of recombinant β-glucosidase with chitin binding domain
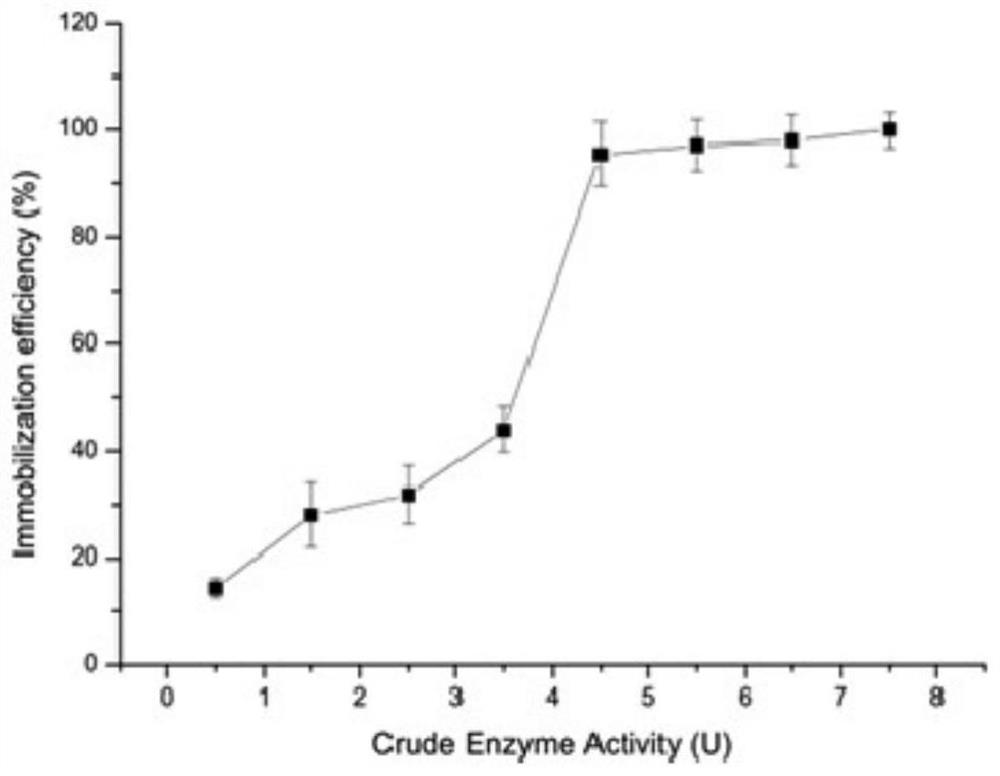
[0059] The recombinant plasmid pLChBD-AcBg prepared in Example 2 was transformed into Escherichia coli C43 (DE3), coated with LB plates containing ampicillin, cultured overnight at 37°C, and colonies in good condition were picked, and positive transformants were verified by PCR. And cultivated in liquid LB medium containing ampicillin, and selected recombinant Escherichia coli strains that meet the requirements. Take the above-mentioned preserved bacteria and inoculate them into 5 mL of liquid LB medium containing ampicillin for overnight culture at 37°C, transfer 100 mL of new LB liquid medium with 1% (v / v) inoculum amount, cultivate to OD600=1.0, add IPTG To a final concentration of 1mM / L, induce culture at 25°C for 24h. Centrifuge at 8000rpm / min for 10min to collect induced expression cells, resuspend the cells in 10mL of 20mM sodium phosphate buffer solution containing 40mM / L NaCl at p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com