Application of a marine Halomonas exopolysaccharide in the preparation of immune enhancer
A technology of Halomonas and exopolysaccharide, which is applied in the preparation of marine Halomonas exopolysaccharide and the field of preparation of immune enhancers, can solve problems such as adverse immune response of immunity, and achieve increased expression and improved immune function. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
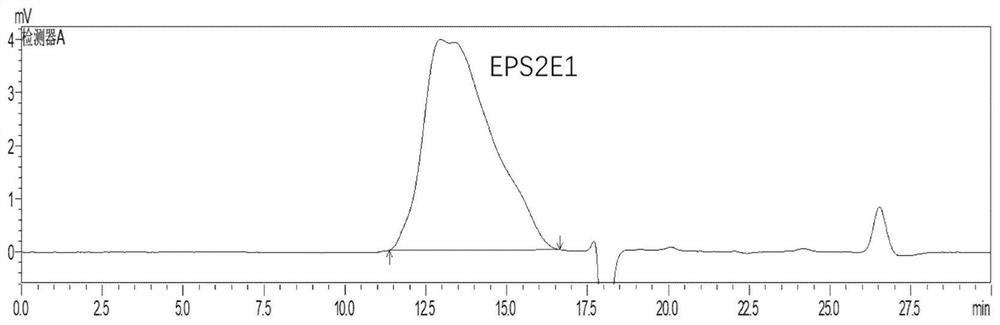
[0029] The preparation method of marine Halomonas exopolysaccharide EPS2E1, the specific steps are as follows:
[0030] 1) Extraction of crude polysaccharide
[0031] Halomonas sp.2E1 was inoculated into 2216E (containing 1% sucrose) medium, placed on a shaker at 28°C, 150rpm for 48h. After the fermentation, centrifuge the Halomonas fermentation broth (4000rpm) for 15min to remove the bacteria, collect the supernatant, add three times the volume of 95% ethanol solution to the supernatant while stirring, stir evenly, and place it in a refrigerator at 4°C for static Set overnight, followed by centrifugation (4000 rpm, 15 min) to collect the precipitate, redissolved in water, dialyzed (molecular weight cut-off is 3.5 kDa), and freeze-dried to obtain crude polysaccharide.
[0032] 2) Purification of exopolysaccharides
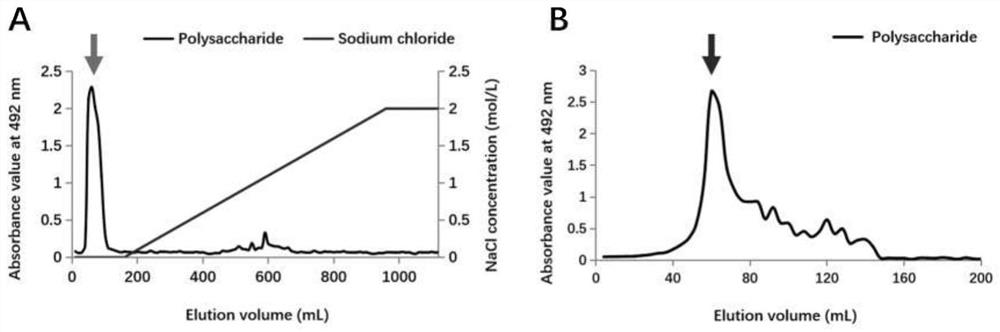
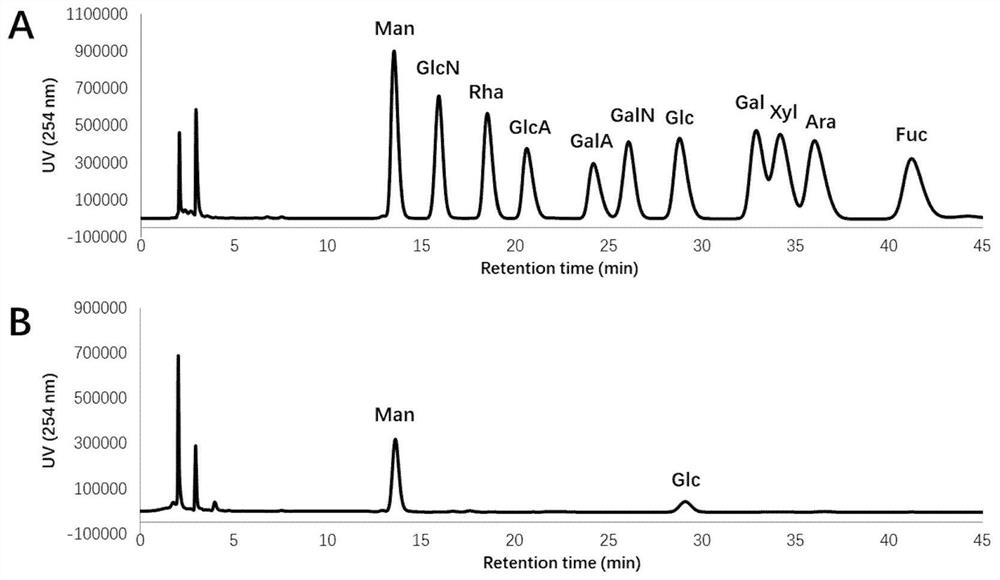
[0033] The crude polysaccharide was purified by DEAE Fast Flow ion-exchange column chromatography. After loading, eluted with pure water for 2 column volumes (CV...
Embodiment 2
[0041] Effects of EPS2E1 administration on the proliferation activity of RAW264.7 cells in vitro
[0042] experimental method:
[0043] Take the RAW264.7 cells in the logarithmic phase of growth, pipetting to make a single cell suspension, and after cell counting, the RAW264. 5 Cells / mL were cultured in a 96-well plate. When the cells grew to about 50%, the old medium was replaced with 100 μL of FBS-free medium, and starved for 12 h. Then, the old medium was replaced with 100 μL blank or medium containing EPS2E1 with different concentrations, and the culture was continued for 24 h. Add 20 μL of MTT solution with a concentration of 5 mg / mL to each well, continue to incubate for 4 h, discard the supernatant, add 150 μL of DMSO to each well, shake gently for 10 min at room temperature to fully dissolve the crystals, and measure the absorbance at a wavelength of 490 nm.
[0044] Cell survival rate=(OD value of experimental group / OD value of blank control group)×100%.
[0045] T...
Embodiment 3
[0047] In vitro administration of EPS2E1 inhibited the secretion of NO secreted by RAW264.7 cells.
[0048] experimental method:
[0049] RAW264.7 cells were placed in 5 × 10 5 Cells / mL were placed in a 96-well plate and cultured in a constant temperature incubator (37°C, 5% CO2). When the cells grew to about 50%, the old medium was replaced with 100 μL of FBS-free medium, and starved for 12 hours. . Add samples according to the following groups: (1) blank control group; (2) LPS treatment group (final LPS concentration 1 μg / mL); (3) EPS2E1 treatment group (final concentration 6.25-200 μg / mL); Placed in an incubator for 24h. Collect the supernatant and measure the NO concentration by the Griess method: take 50 μL of the supernatant and add 50 μL of Griess reagent I and II solutions in turn, mix well and place at room temperature for 10 min to fully react, measure the absorbance at a wavelength of 540 nm, and use sodium nitrite The solution was a standard solution to establi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com