Method for synthesizing and preparing amantadine dry product
A technology of amantadine dry product and amantadine, which is applied in the preparation of nitro compounds, amino compounds, organic compounds, etc., can solve the problem of raw material selection in the production process of proportional pollution, and achieve high conversion rate and equipment requirements The effect of simplicity and mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
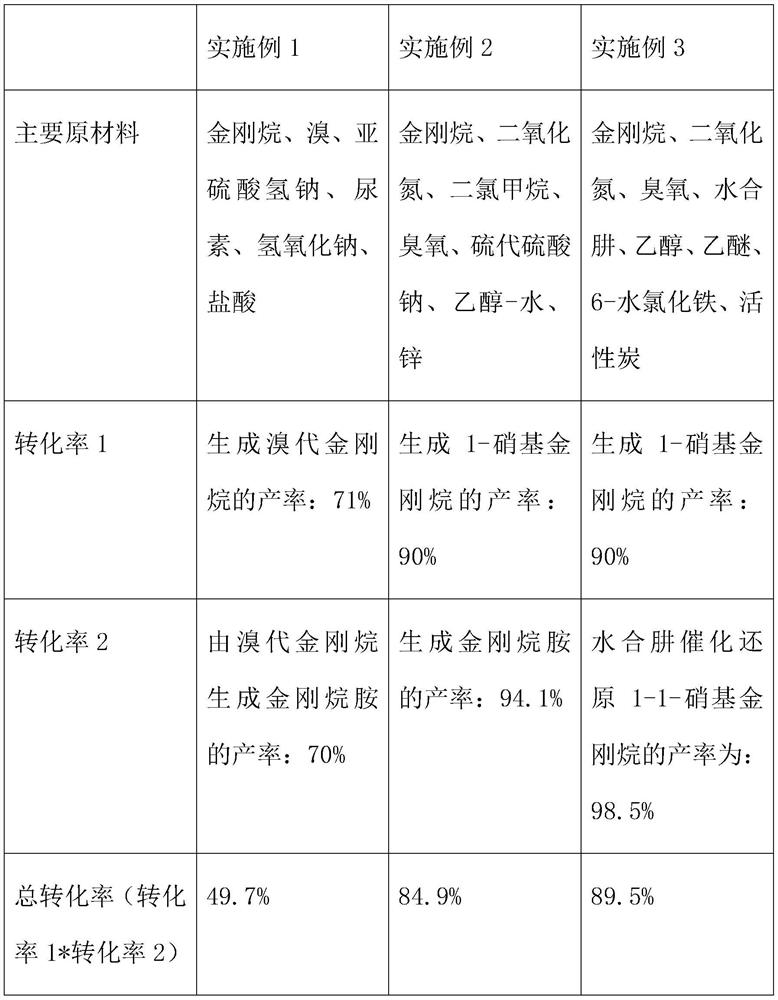
[0058] A kind of amantadine dry product synthetic preparation method, its synthetic raw material comprises adamantane, bromine, sodium bisulfite, urea, sodium hydroxide and hydrochloric acid, comprises the steps:
[0059] A1: Synthesis of bromoadamantane;
[0060] A11: Raw material preparation, take adamantane: bromine: sodium bisulfite in a mass ratio of 1:2.2:0.56;
[0061] A12: Grind the adamantane and add it to the pre-dried flask, gradually drop into the liquid bromine under the condition of stirring, and heat slowly;
[0062] A13: Overnight after the reaction, gradually heat to 45 degrees Celsius the next day, and add dropwise a 7% solution prepared by sodium bisulfite;
[0063] A14: filter, wash the filter cake with water until the pH is 7, and dry naturally to obtain bromoadamantane;
[0064] A2: Synthesis of dry amantadine from bromoadamantane;
[0065] A21: Mix bromoadamantane and urea in a ratio of 1:0.45, and heat at a fixed temperature;
[0066] A22: Cool down...
Embodiment 2
[0071]A method for synthesizing and preparing amantadine dry product, its main synthetic raw materials include adamantane, nitrogen dioxide, methylene chloride, ozone, sodium thiosulfate, ethanol-water and zinc, comprising the following steps:
[0072] B1: Synthesis of nitro compounds;
[0073] B11: Add adamantane and dichloromethane into the flask according to the ratio of 1g:120ml, and stir at a certain temperature;
[0074] B12: Introduce 30 equivalents of nitrogen dioxide under the condition of -70 to -80 degrees Celsius,
[0075] B13: Introduce ozone at a low speed and react for 30 minutes;
[0076] B14: subsequent addition of sodium bicarbonate solution, subsequent washing of the organic phase to neutrality,
[0077] B15: The product 1-nitroadamantane can be obtained by rotary evaporation after drying;
[0078] B2: Synthesis of amantadine;
[0079] B21: Take another flask, add ethanol-water mixed solution, glacial acetic acid, zinc powder and ammonium chloride in seq...
Embodiment 3
[0091] A method for synthesizing and preparing dry amantadine, the main raw materials of which include adamantane, nitrogen dioxide, ozone, hydrazine hydrate, ethanol, ether, ferric chloride 6-hydrate and activated carbon, characterized in that it comprises the following steps:
[0092] C1: Synthesis of nitro compounds;
[0093] C11: Add adamantane and dichloromethane into the flask according to the ratio of 1g:120ml, and stir at a certain temperature;
[0094] C12: Introduce 30 equivalents of nitrogen dioxide under certain conditions,
[0095] C13: Introduce ozone at a low speed and react for 30 minutes;
[0096] C14: sodium bicarbonate solution is then added, the organic phase is then washed to neutrality,
[0097] C15: After drying, the product 1-nitroadamantane can be obtained by rotary evaporation;
[0098] C2: preparation of catalyst;
[0099] C21: Add ethanol, ether and ferric chloride 6-hydrate into the flask according to the ratio of 1ml: 5ml: 0.15g;
[0100] C22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com