Application of oral fullerene material in preparation of medicine for preventing and/or treating myocardial ischemia-reperfusion injury or ischemic heart disease
A technology for reperfusion injury and myocardial ischemia, applied in the field of biomedicine, can solve the problems of low clinical accessibility and difficult operation, and achieve the effect of high clinical accessibility, good biocompatibility and remarkable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the preparation of fullerene material
[0079] Take an appropriate amount of fullerene C60 powder with a particle size of 2-3 μm, mix it evenly with pharmaceutical excipients such as microcrystalline cellulose, copovidone, glyceryl behenate, etc., and press it into tablets of 200 mg each with a tablet machine.
[0080] When used in animal experiments, the tablet is dissolved in purified water to form a suspension for use.
Embodiment 2
[0081] Embodiment 2: Particle size characterization of fullerene materials
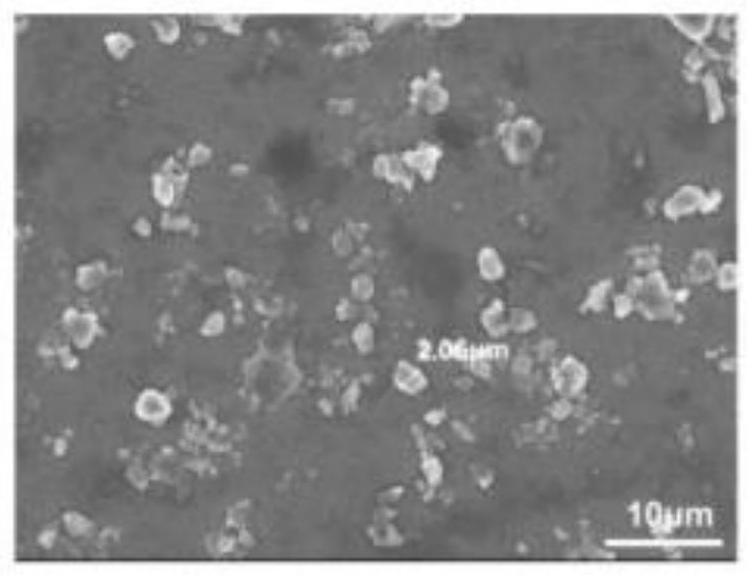
[0082] Take an appropriate amount of the fullerene C60 material prepared in Example 1 and drop it on the cleaned silicon wafer. After the water is completely evaporated, observe the morphology and particle size of the C60 material with a scanning electron microscope SEM (Hitachi, S-4800, Japan). .
[0083] Experimental results such as figure 1 As shown, the particle size of the C60 material is 2-3 μm.
Embodiment 3
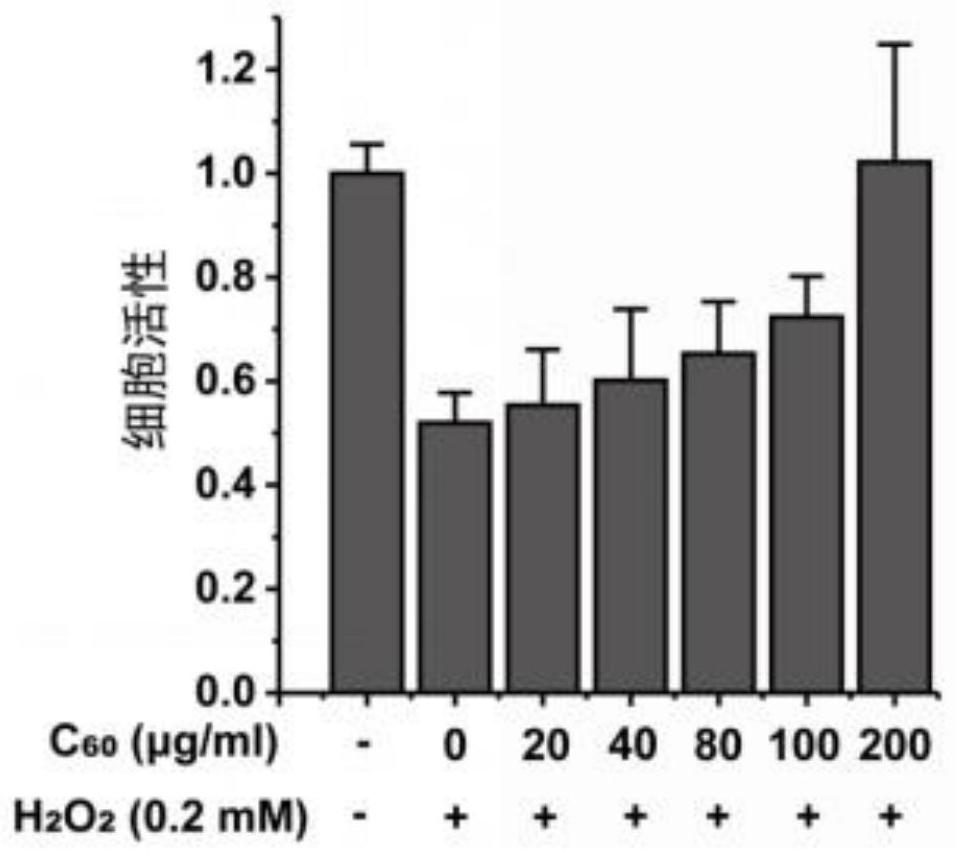
[0084] Example 3: Cytotoxicity and oxidative damage repair effect of fullerene materials on human umbilical vein endothelial cells HUVEC
[0085] 1. Cell culture conditions: HUVEC cells were cultured in a cell culture incubator (37°C, 5% carbon dioxide), the medium was MEM, and 10% FBS and 1% penicillin-streptomycin double antibody solution were added.
[0086] 2. Experimental methods and results:
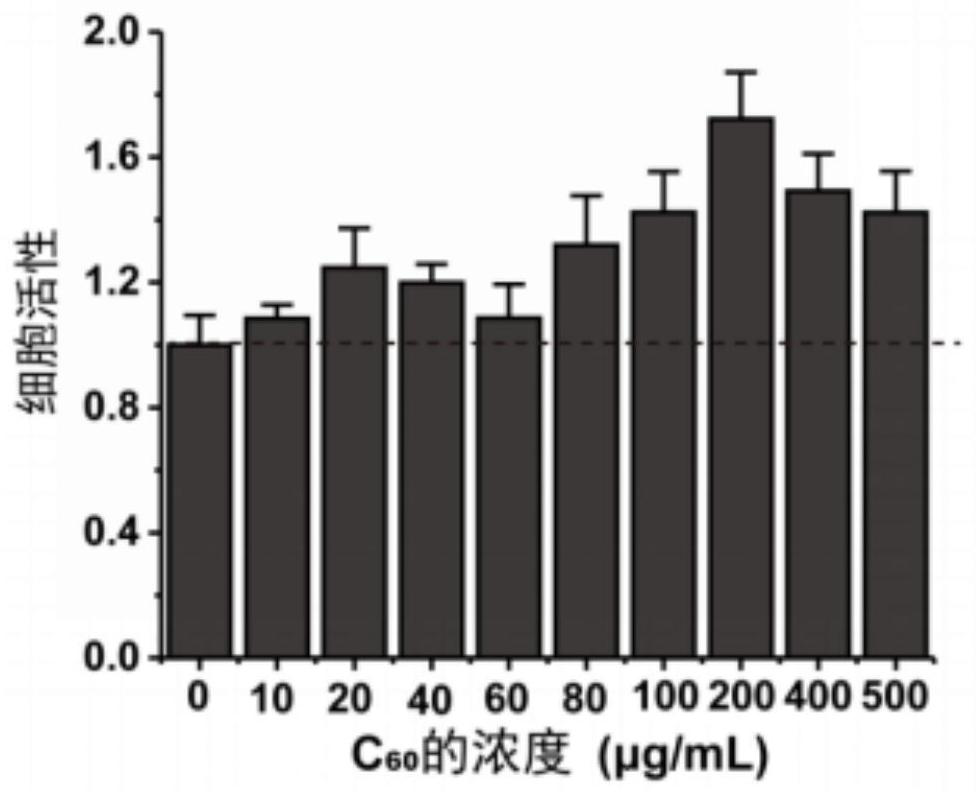
[0087] (1) Use the WST-8 method to detect whether the fullerene material has cytotoxicity
[0088] Inoculate the HUVEC cell suspension with a density of 5×10^4 / ml in a 96-well plate at 200 μl / well. After culturing for 24 hours, suck out the supernatant and replace it with 200 μl of fullerene materials with different concentrations to make the final concentration 0 , 10, 20, 40, 60, 80, 100, 200, 400, 500μg / ml, 6 parallel experiments for each group, cultured for 24h. Add 100 μl of CCK-8 solution (diluted in colorless DMEM) to each well, and test the OD values of different groups...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com