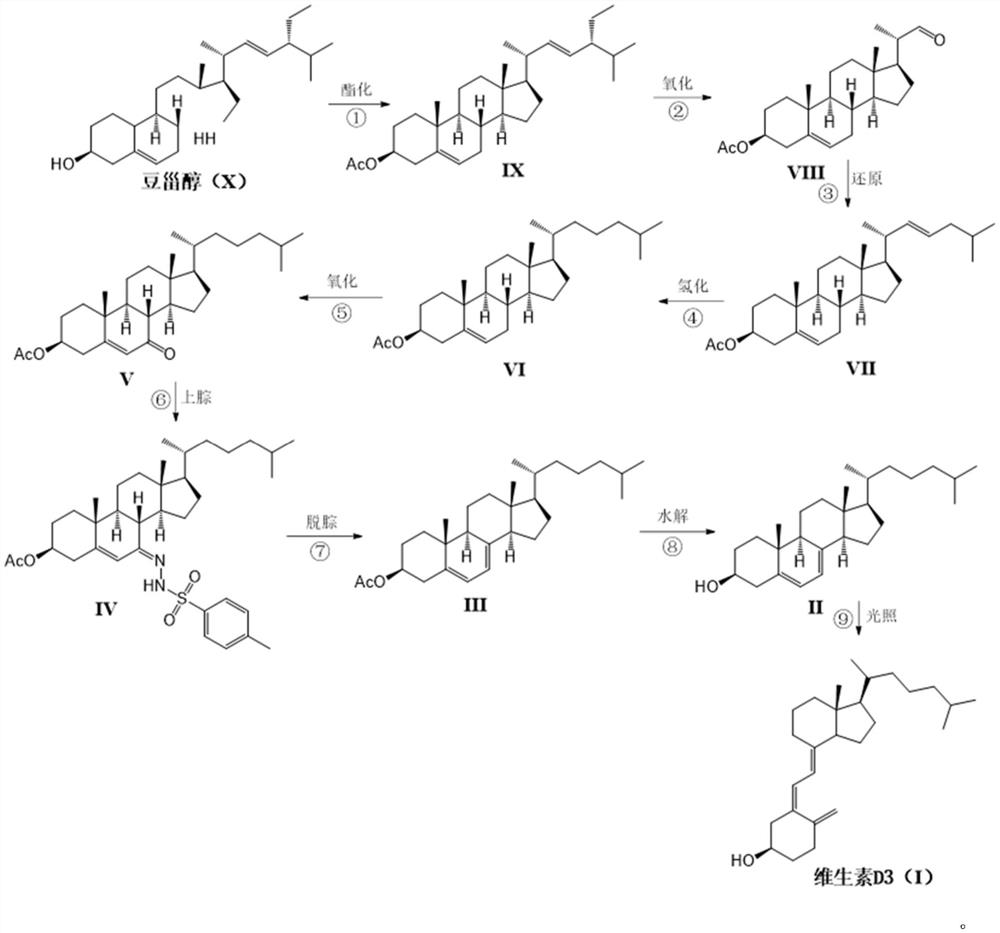
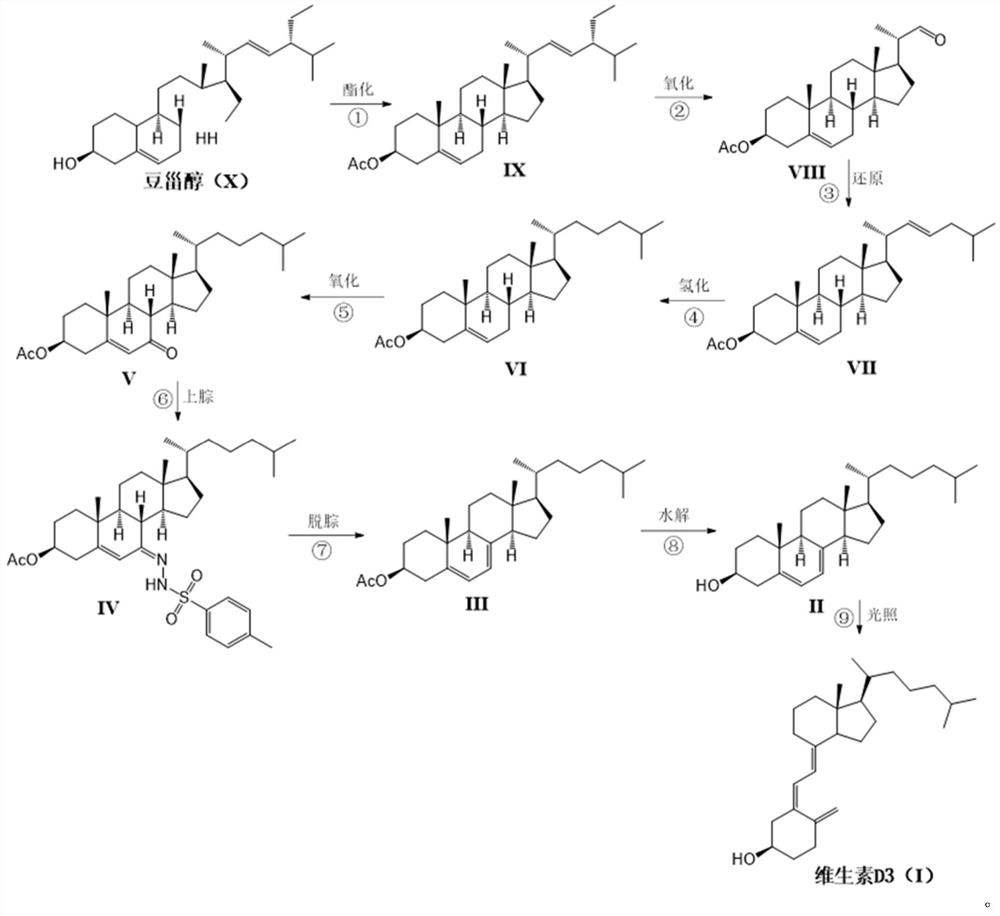
Novel industrial method for preparing vitamin D3 by taking stigmasterol as raw material
A vitamin and stigmasterol technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of high cost, environmental protection, and low cholesterol yield, and achieve the effect of mild reaction conditions, strong operability, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 stigmasterol acetate (IX)
[0036] 10Kg stigmasterol was added to a 1000L enamel reaction kettle, 200g pyridine and 3.2Kg powdered potassium carbonate were added. Close the enamel kettle, vacuum inhale 200L n-hexane. The stirring was started, and the jacket was heated to an internal temperature of 60°C. 2.96Kg of acetic anhydride was added dropwise while maintaining the temperature. After the dropwise addition was completed, the temperature was maintained to continue the reaction for 3 hours. After the reaction was judged to be complete by thin layer chromatography. Filter while hot with a stainless steel plate frame, rinse the filter cake with 40 L of fresh n-hexane, and concentrate the combined n-hexane solution to dryness in vacuo to obtain a white solid. The white solid was added with 100L of ethanol, refluxed at 80°C, dissolved and cleared, cooled to crystallize, and 10.8Kg of compound IX was precipitated as a white needle produc...
Embodiment 2
[0037] The preparation of embodiment 2 side chain oxide compound (VIII)
[0038] Add 10Kg of compound IX to 500L of ethanol, use a cold tank machine to cool down to -50°C, and pass through oxygen (containing 1% ozone, which is freshly produced by an ozone machine) under stirring conditions for oxidation; TLC tracking until the oxidation is complete; oxidation After completion, replace oxygen with nitrogen; let the system slowly heat up to 0°C;
[0039] Add 2000g of thiourea to 20L of 90% ethanol water, dissolve and clear, then slowly add to the oxidation reaction system; slowly rise to room temperature and stir for 16 hours; after the reaction is complete, filter to remove the precipitate; wash the precipitate with 2000ml of 90% ethanol water ; The combined filtrates were concentrated at 60°C until the volume reached 50 L, and cooled to crystallize; 6.4Kg of white solid compound VIII was obtained by filtration, with a yield of 78%.
[0040] If an additional 5 Kg of silica gel...
Embodiment 3
[0041] Preparation of Embodiment 3 Side Chain Isopentyl Reductant (VII)
[0042] Add 5.85Kg of triphenylphosphine to 120L of toluene and dissolve it clearly; dissolve 3.39Kg of 1-bromoisopentane in 4.5L of toluene; Add to the triphenylphosphine solution; heat up and stir until reflux reaction; after the reaction is complete, cool down to room temperature; add 2.85Kg sodium tert-butoxide, and continue to stir;
[0043] Dissolve 5.58Kg of compound VIII in 6L of toluene and add it dropwise into the above reaction system; reflux the reaction again, monitor by TLC until the reaction of the raw materials is completed; cool in an ice-water bath, add 10% phosphoric acid water dropwise after cooling, and adjust the pH value to neutral Properties; liquid separation, the toluene layer was concentrated to dryness, refluxed with 75L of methanol to dissolve, left to cool and crystallized naturally to obtain 5.61Kg of compound VII, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com