Thermostable difunctional glutathione synthetase mutant and application thereof
A technology of glutathione and mutants, which is applied in the field of bioengineering, can solve the problems of few large-scale practical applications, and achieve the effects of increasing production, synthesis efficiency, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
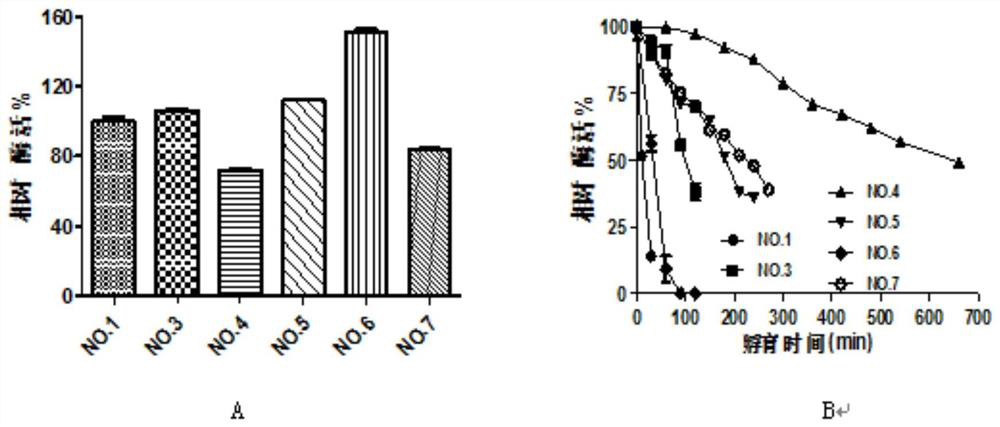
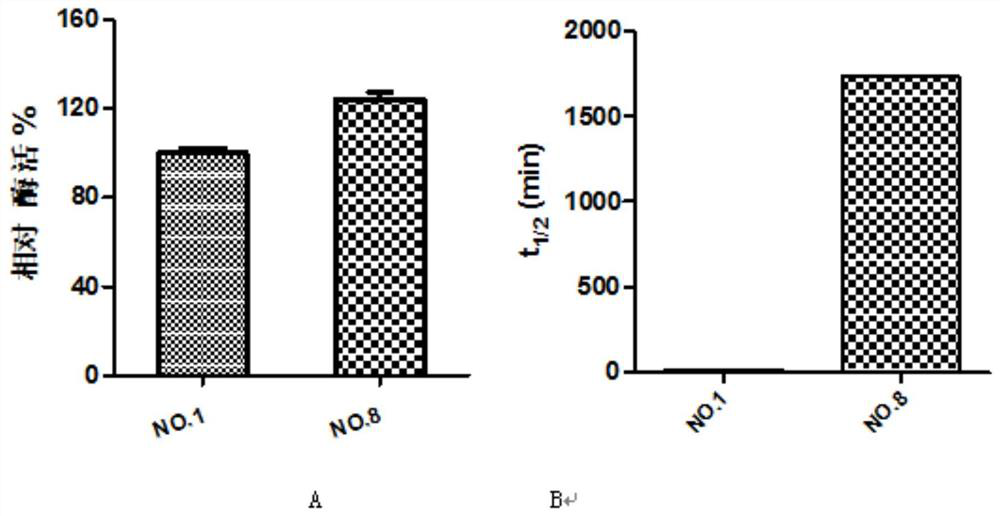
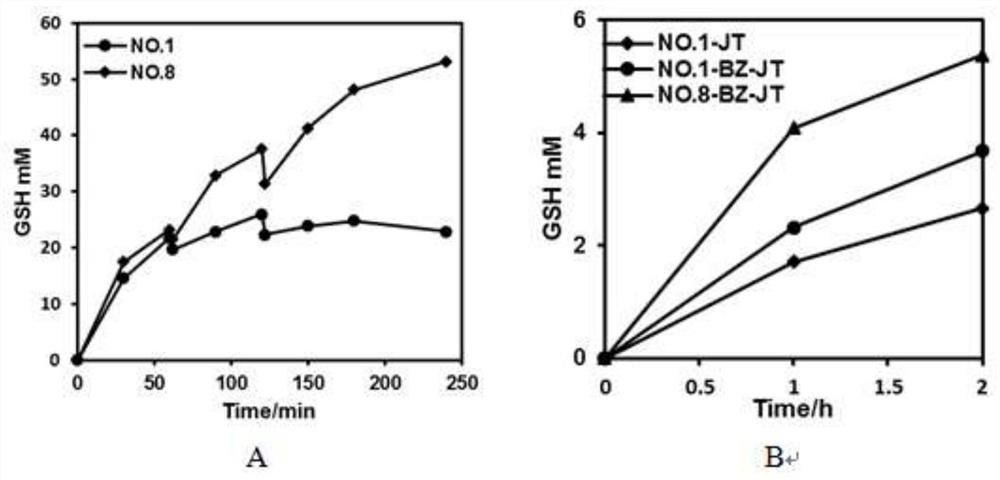
[0019] Example 1: DNA recombination of bifunctional glutathione synthetase from different sources, construction of recombinant expression plasmids and engineering bacteria
[0020] In this embodiment, the bifunctional glutathione synthase gene (shown in sequence table SEQ ID NO.1 and SEQ ID NO.2) derived from Streptococcus sanguinis (gshFss) and S.thermophilus (gshFst) was selected. The first gene was selected from SEQ ID NO.4 and SEQ ID NO.7 in Chinese patent CN102071171B, and the gshFsa whose crystal structure had been reported was used as a template for homologous sequence comparison to find the corresponding γ-GCS, γ-GS and intermediate The functional domains of the linker domains were combined in different ways to obtain mutants that can improve thermal stability. The specific primer sequences are shown in Table 1, and the sequences were sent to Shanghai Bioengineering Co., Ltd. for synthesis.
[0021] Table 1 Sequence list of DNA recombination mutation primers
[0022] ...
Embodiment 2
[0026] Example 2: Site-directed mutagenesis of bifunctional glutathione synthetase, recombinant expression plasmid and construction of engineering bacteria
[0027] Through the calculation of ΔΔG, some potential mutation points that can improve the thermal stability of the enzyme can be screened. In the present invention, the ΔΔG of different mutation points is calculated by software such as ROSETTA and FoldX, and amino acid sites with lower ΔΔG values (usually unstable amino acid positions) are selected for mutation by comparison. The specific primer sequences are shown in the table 2. The sequence was sent to Shanghai Bioengineering Co., Ltd. for synthesis.
[0028] Table 2 Design of primers for site-directed mutagenesis
[0029]
[0030]Preparation of linearized carrier: the method is the same as that of the linearized carrier in Example 1
[0031] Site-directed mutagenesis: use the recombinant as a template and perform amplification with the corresponding primers in...
Embodiment 3
[0034] Example 3: Protein expression and protein purification of different mutant strains
[0035] The different recombinant bacteria obtained in Example 1 and Example 2 were inoculated into 50ml LB containing 50μg / ml Kanamycin, 37°C, 220rpm shaking culture for 6-8h, and then the bacteria were transferred to 100ml LB, 37°C, Cultivate to OD at 220rpm 600 =0.6-0.8, add IPTG with a final concentration of 0.2mM and induce at 18°C and 220rpm for 16h. Centrifuge at 6000rpm for 10min to collect the bacteria.
[0036] The protein was purified by Ni-NTA nickel column affinity chromatography. Resuspend the bacteria with 50ml of buffer solution (20mM Tris-HCl; 250mMNaCl; 10mM imidazole), then crush it with a pressure breaker, centrifuge at 8000rpm at 4°C for 20min, take the supernatant and load it on nickel that has been equilibrated with Tris-HCl buffer On the column, the adsorbed protein was gradiently eluted with loading buffer containing different concentrations of imidazole (0-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com