Recycling method of fluorine-containing sulfuric acid waste liquid
A sulfuric acid waste liquid and recycling technology, which is applied in the field of resource recycling of fluorine-containing sulfuric acid waste liquid, can solve problems such as difficult separation of calcium sulfate and calcium fluoride, waste of resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
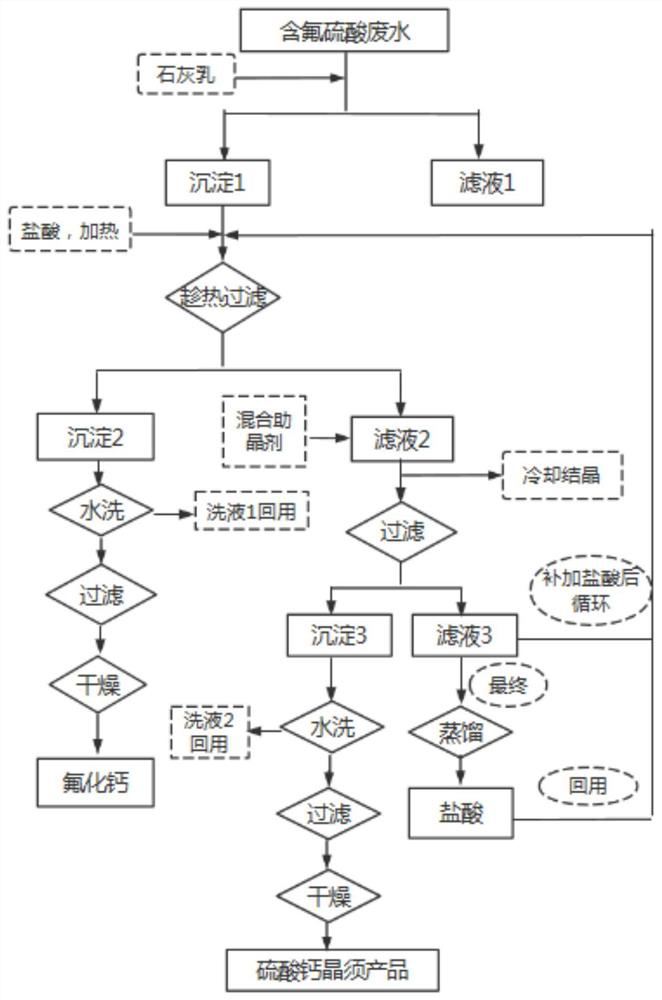
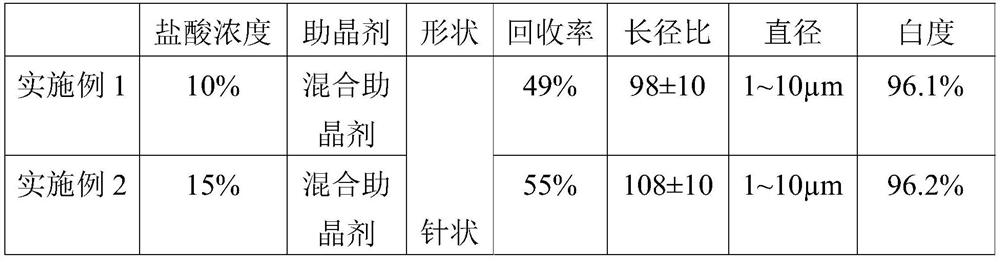
[0035] Take 1000mL of a fluorine-containing sulfuric acid waste liquid (fluorine content 12g / L, sulfuric acid content 30%), add milk of lime to the waste liquid, adjust the pH of the solution to 8, stir well and then filter to obtain a filter cake, which is calcium fluoride and calcium sulfate mixture. Add 10wt% hydrochloric acid to the mixture of calcium fluoride and calcium sulfate with a mass volume ratio of 1:0.02g / L, then heat and stir at 80°C, then add 0.675g of mixed crystal aid (stearic acid: magnesium chloride mass The ratio is 1:1), after stirring for 1 hour, filter while hot, the obtained filter cake is washed with water, filtered, and dried to obtain 22.62g of calcium fluoride product, the filtrate is cooled at room temperature for 4 hours, filtered, the obtained filter cake is washed with water, filtered , and dried at 50°C to obtain 313.47g of calcium sulfate whisker product.
Embodiment 2
[0037] Take 1000mL of a fluorine-containing sulfuric acid waste liquid (fluorine content 12g / L, sulfuric acid content 30%), add milk of lime to the waste liquid, adjust the pH of the solution to 8, stir well and then filter to obtain a filter cake, which is calcium fluoride and calcium sulfate mixture. Add 15wt% hydrochloric acid to the mixture of calcium fluoride and calcium sulfate with a mass volume ratio of 1:0.02g / L, then heat and stir at 80°C, then add 0.675g of mixed crystal aid (stearic acid: magnesium chloride mass The ratio is 1:1), after stirring for 1 hour, filter while hot, the obtained filter cake is washed with water, filtered, and dried to obtain 23.17g of calcium fluoride product, the filtrate is cooled at room temperature for 4 hours, filtered, the obtained filter cake is washed with water, filtered , dried at 50°C to obtain 351.85g of calcium sulfate whisker product.
Embodiment 3
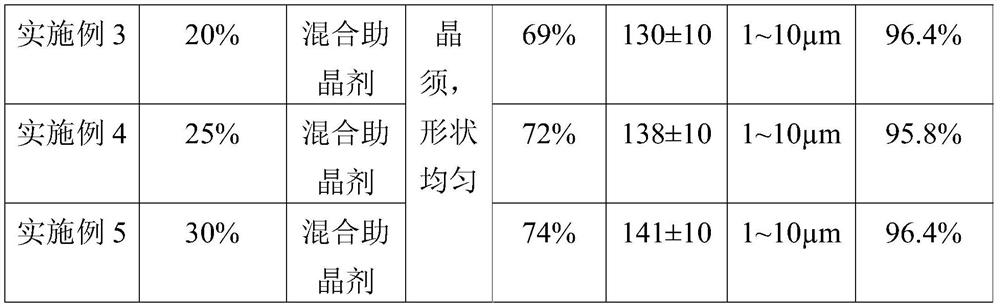
[0039]Take 1000mL of a fluorine-containing sulfuric acid waste liquid (fluorine content 12g / L, sulfuric acid content 30%), add milk of lime to the waste liquid, adjust the pH of the solution to 8, stir well and then filter to obtain a filter cake, which is calcium fluoride and calcium sulfate mixture. Add 20wt% hydrochloric acid to the mixture of calcium fluoride and calcium sulfate with a mass volume ratio of 1:0.02g / L, then heat and stir at 80°C, then add 0.675g of mixed crystal aid (stearic acid: magnesium chloride mass The ratio is 1:1), after stirring for 1 hour, filter while hot, the obtained filter cake is washed with water, filtered, and dried to obtain 22.36g of calcium fluoride product, the filtrate is cooled at room temperature for 4 hours, filtered, the obtained filter cake is washed with water, filtered , dried at 50°C to obtain 441.41g of calcium sulfate whisker product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com