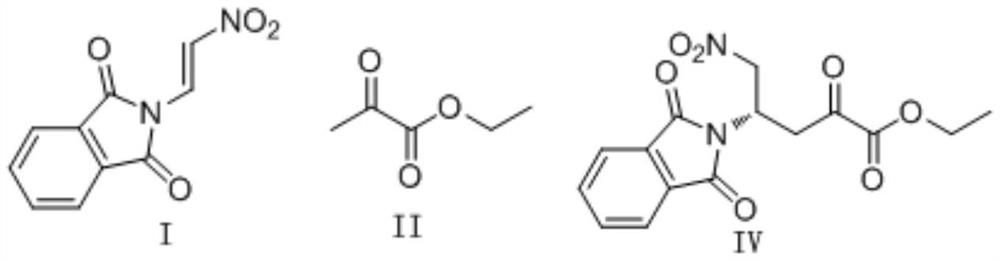
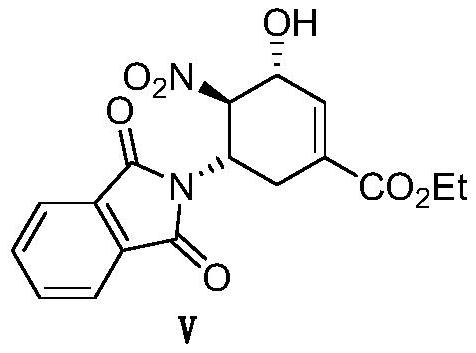
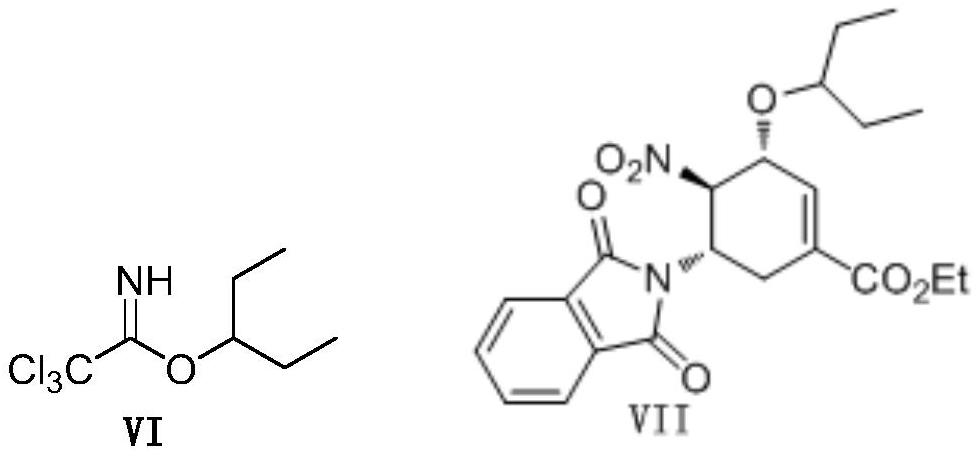
Novel synthesis method of oseltamivir
A new synthesis and compound technology, applied in the field of drug synthesis, can solve the problems of unsatisfactory total yield and long steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0094] The preparation method of hydrogen bond catalyst 1-((1R,2R)-2-aminocyclohexyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea (Ⅲ-1), comprising the steps of:
[0095] Add (1R,2R)-1,2-cyclohexanediamine (910.0 mg, 8.0 mmol) into 40 mL of anhydrous tetrahydrofuran, and then add 3,5-bis(tri Fluoromethyl)phenyl isothiocyanate (1.46mL, 8.0mmol), the dropwise addition time is 45 minutes; Silica gel column chromatography for purification, the eluent is a mixed solvent of dichloromethane and methanol, wherein the volume ratio of dichloromethane to methanol is 100:1 to 20:1;
[0096] A white flaky solid product (2.81 g, yield 91%) was obtained as 1-((1R,2R)-2-aminocyclohexyl)-3-(3,5-bis(trifluoromethyl)phenyl ) Thiourea (III-1). References: Liu, Y.; Kang, T.R.; Liu, Q.Z.; Chen, L.M.; Wang, Y.C.; Liu, J.; Xie, Y.M.; Yang, J.L.; -6093.
[0097] The reaction equation is as follows:
[0098]
preparation example 2
[0100] Hydrogen bond catalyst (R)-1-(2'-amino-[1,1'-binaphthyl]-2-yl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea ( Ⅲ-2) preparation method, comprising steps:
[0101] Under argon atmosphere, (R)-(+)-1,1'-bi-2-naphthylamine (610.0 mg, 2.16 mmol) was added to 9 mL of dry CH 2 Cl 2 3,5-bis(trifluoromethyl)phenyl isothiocyanate (329.0mg, 1.8mmol) was added dropwise to the above system under stirring at 0°C for 45 minutes; After that, it was raised to room temperature, and stirred and reacted at room temperature for 4 hours; after the reaction was completed, the solvent was distilled off under reduced pressure, and the resulting product was purified by silica gel column chromatography, and the eluent was petroleum ether at a volume ratio: ethyl acetate=10:1 The solvents were mixed to obtain a light yellow solid product (590.0 mg, yield 63%), namely (R)-1-(2'-amino-[1,1'-binaphthyl]-2-yl)-3- (3,5-bis(trifluoromethyl)phenyl)thiourea (III-2). References: Galzerano, Patrizia; Benci...
preparation example 3
[0105] Hydrogen bond catalyst 1-((1R,2R)-2-amino-1,2-diphenylethyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea (Ⅲ-4) The preparation method comprises steps:
[0106] Add (1R,2R)-1,2-diphenylethylenediamine (910.0mg, 8.0mmol) into 10mL tetrahydrofuran, then add 3,5-bis(trifluoromethyl) dropwise under stirring at 0°C Phenyl isothiocyanate (1.46mL, 8.0mmol), the dropwise addition time was 45 minutes; after the dropwise addition, the reaction system was raised to room temperature, and the reaction was stirred at room temperature for 4h; the reaction liquid was distilled off the solvent under reduced pressure, and the obtained product Purify by silica gel column chromatography, the eluent is a mixed solvent of dichloromethane and methanol, wherein the volume ratio of dichloromethane to methanol is 30:1 to 20:1, to obtain a white flaky solid (2.23g, harvested rate 83%), which is 1-((1R,2R)-2-amino-1,2-diphenylethyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea ( III-4). References:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com