Method for detecting related substances of pramipexole dihydrochloride sustained-release tablets
A technology for the detection of pramipexole hydrochloride and its detection method, which is applied in the field of detection of related substances in pramipexole hydrochloride sustained-release tablets, which can solve the problems of inability to accurately quantify impurities, cumbersome preparation steps, and easy hydrolysis of impurities, and achieve simplified preparation and simple operation , good quality control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Adopt the import registration standard method of pramipexole hydrochloride sustained-release tablets:
[0063]Chromatographic conditions and system suitability test: use InertsilODS-3, (3.0mm×150mm, 3μm) as filler; mobile phase A is phosphate buffer (take sodium octane sulfonate 5.0g and potassium dihydrogen phosphate 9.1g, Add 1000ml of water to dissolve, adjust the pH value to 3.0 with phosphoric acid), mobile phase B is acetonitrile; carry out gradient elution according to Table 1, the flow rate is 0.5ml per minute; the column temperature is 30 ° C; use a diode array detector to detect, the detection wavelength 240nm, 262nm and 326nm respectively.
[0064] Table 1
[0065]
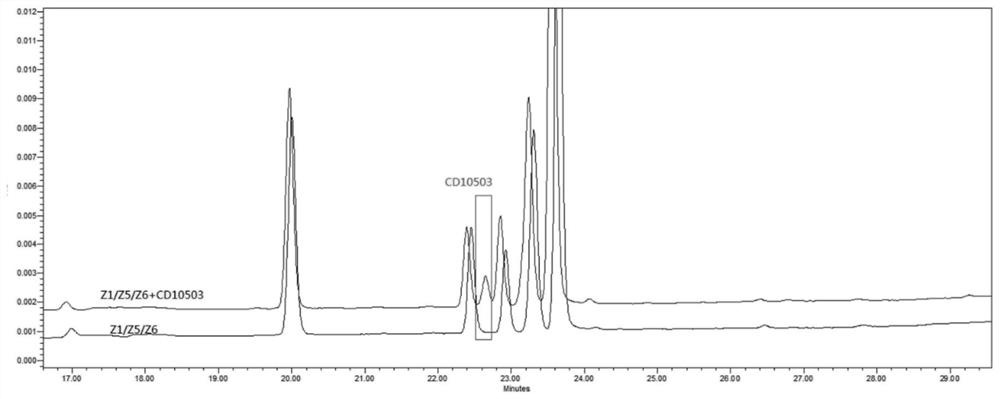
[0066] Accurately weigh the impurity PMH-Z5 and PMH-Z6 reference substance mixture, PMH-Z1, PMH reference substance appropriate amount, add solvent (1) to dissolve, dilute with phosphate buffer (pH2.0) and make every 1ml containing PMH Solutions of about 0.15 μg, 0.15 μg, 0.15 μg and 15 μg e...
Embodiment 2
[0070] Embodiment 2 method screening
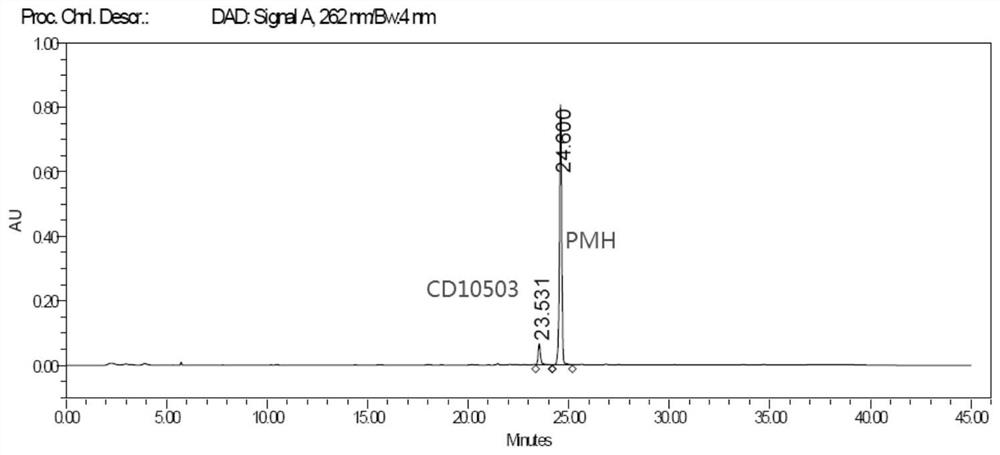
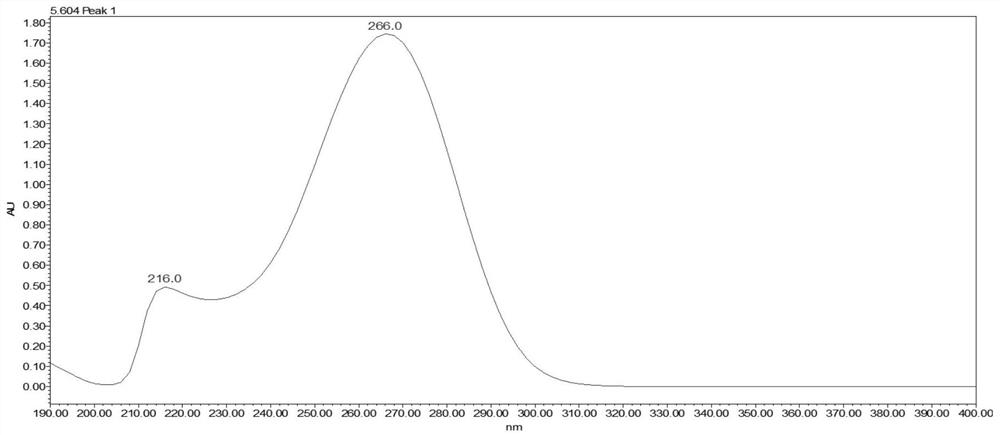
[0071] Perform wavelength scanning on CD10503 and PMH, the results are shown in image 3 , Figure 4 , CD10503 has a maximum absorption at 266nm, and PMH has a maximum absorption at 262nm.
[0072] Condition 1:
[0073] (1) Chromatographic conditions:
[0074] Table 2
[0075] Chromatographic column CHIRALPAK AD-H, 250×4.6mm, 5.0μm (L-S-67) mobile phase: n-Hexane-Ethanol-Diethylamine (850:150:1) flow rate 1.0ml / min Column temperature 35℃ Injection volume 75μl Thinner ①Mobile phase②N-hexane-dichloromethane (85:15) wavelength 262nm operation hours 20min
[0076] (2) Solution preparation:
[0077] PMH reference substance solution: Take about 10 mg of PMH raw material drug, add diluent ① to dissolve and dilute to 100 ml.
[0078] CD10503 reference substance solution: Take 3.81mg of CD10503 and add diluent ① to dissolve and dilute to 10ml.
[0079] CD10503 reference substan...
Embodiment 3
[0117] Embodiment 3 method verification
[0118] Best Analysis Method:
[0119]Accurately weigh an appropriate amount of pramipexole hydrochloride sustained-release tablet fine powder (approximately equivalent to 1mg pramipexole hydrochloride), put it in a 10ml measuring bottle, add diluent [n-hexane-isopropanol-ethyl acetate-diethylamine (600 : 300: 100: 1)] appropriate amount, shake to disperse, ultrasonically add diluent to quantitatively dilute to the scale, shake well, filter, take the subsequent filtrate as the test solution; in addition, accurately weigh an appropriate amount of CD10503 reference substance, add dilution The reagent was dissolved and quantitatively diluted to a solution containing about 1 μg per 1 ml, which was used as the reference solution. According to high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition Sibu general rule 0512), with cellulose-tris(3,5-dichlorophenylcarbamate) silica gel as filler (CHIRALPAK IC, 250mm×4.6mm, 5μm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com