A kind of crystal glue for hemostasis and carrying anticancer drugs and preparation method thereof
A technology of anticancer drugs and crystal gel, which is applied in drug delivery, pharmaceutical formulations, applications, etc., and can solve the problems of lack of hemostatic items and lack of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The invention discloses a crystal glue for hemostasis and carrying anticancer drugs, and the preparation method comprises the following steps:
[0040] (1) Synthesize complex A through a double bond functionalization reagent and a first biomacromolecule;
[0041] (2) Synthesize complex B through polyphenolic compounds and second biomacromolecules;
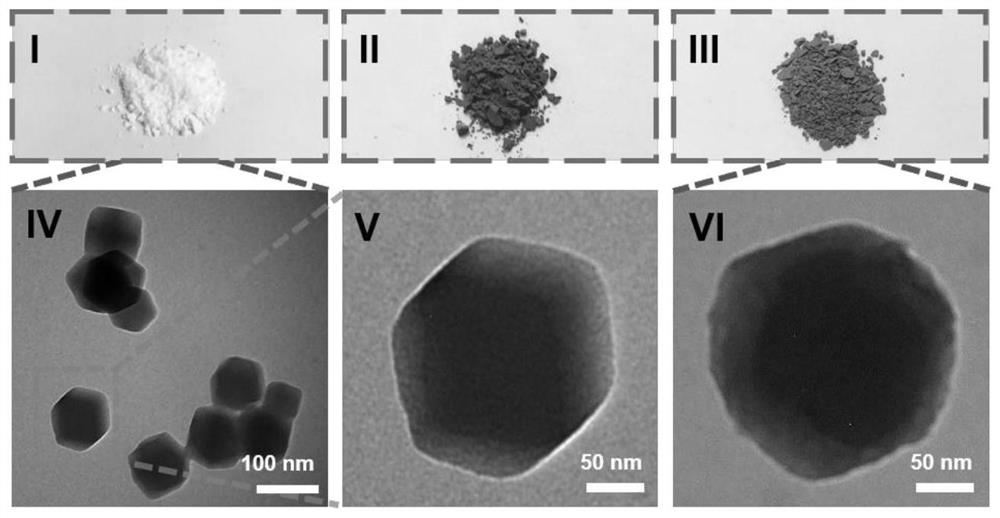
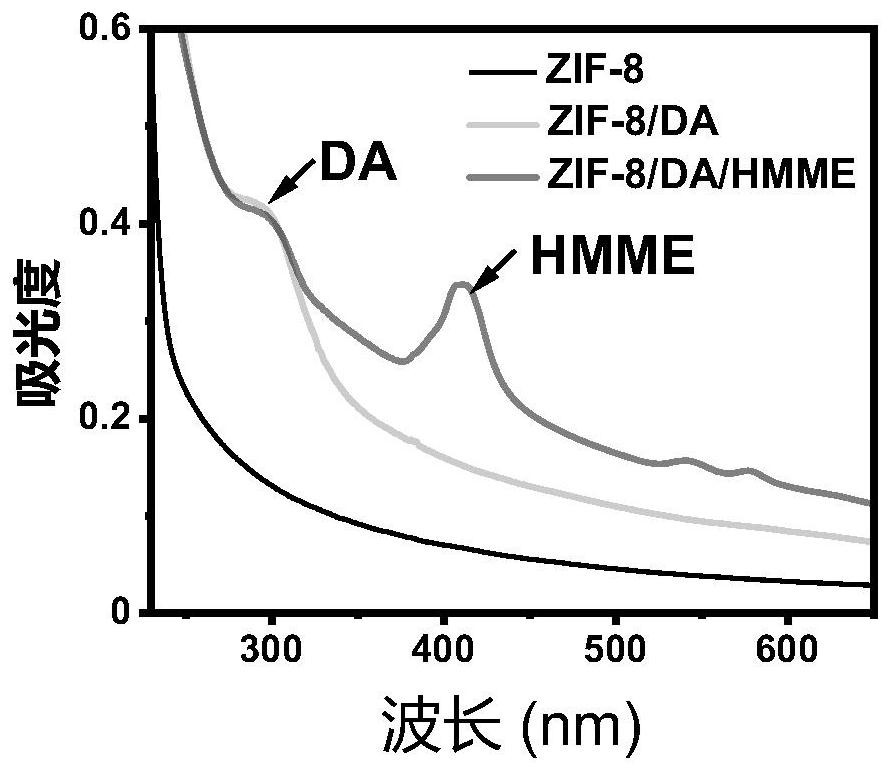
[0042] (3) Synthesis of nanoparticles C by anticancer substances, dopamine and ZIF-8;
[0043] (4) The crystal gel is prepared by compound A, compound B and nanoparticle C.
[0044] Preferably, in step 1, the first biomacromolecule is chitosan or its derivatives, gelatin or its derivatives, hyaluronic acid or its derivatives, alginate or its derivatives, dextran or its derivatives derivatives, cellulose or its derivatives, polyvinyl alcohol or its derivatives, polyethylene glycol or its derivatives, or Pluronic F127 or its derivatives; the first biological macromolecule is a or a combination of them;
[0045] The double ...
Embodiment 1
[0061] (1) Compound A, synthesis of quaternized chitosan (QCSG) modified with glycidyl methacrylate;
[0062] In step (1), the preparation steps of quaternized chitosan modified with glycidyl methacrylate include:
[0063] (1A) Suspend 1 g of chitosan in 36 mL of deionized water to obtain a suspension of chitosan;
[0064] (1B) Add 180 μL of glacial acetic acid to the chitosan suspension obtained in step (1A), and stir at 55° C. for 30 min;
[0065] (1C) The chitosan-glacial acetic acid solution obtained in step (1B) was added with GTMAC with a molar ratio of 2:1 to amino groups on the chitosan backbone under continuous stirring, and the reaction mixture was stirred at 55° C. for 15 hours;
[0066] (1D) After the reaction of step (1C) is completed, under continuous stirring at 55°C, GMA is added dropwise to the above reaction mixture, and the ratio of GMA to amino groups on the main chain of pure chitosan is fixed to 0.5:1.0, under dark conditions The reaction was carried ou...
Embodiment 2
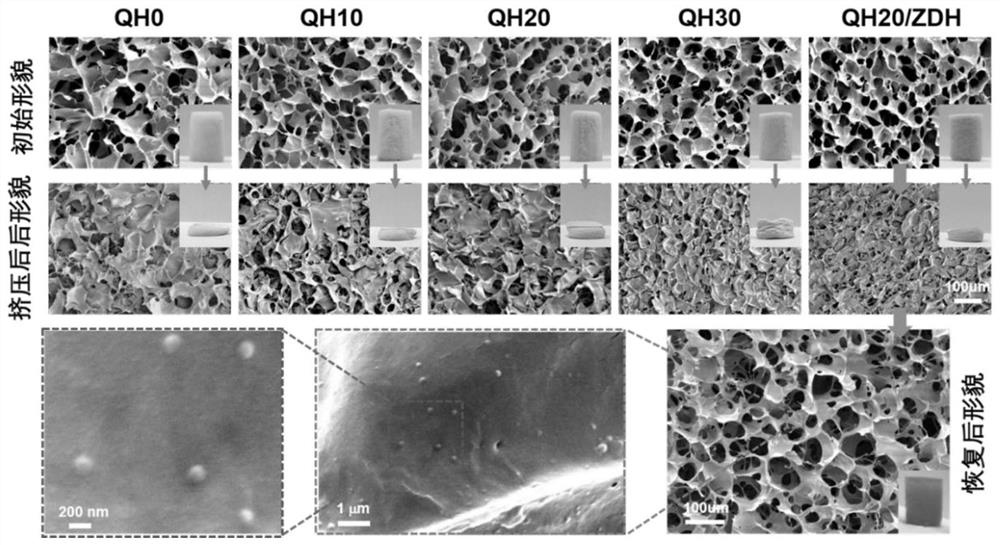
[0087] Different from Example 1, in step (4A), QCSG, HA-DA and ZDH nanoparticles were prepared after 5 wt %, 1.5 wt % and 1 wt % aqueous solution or dispersion, respectively. 600 μL of QCSG aqueous solution was mixed with 100 μL of ZDH aqueous dispersion and 200 μL of HA-DA aqueous solution, pre-cooled in an ice bath, and the corresponding prepared crystal gel was named QH10 / ZDH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com