Method for synthesizing terephthalonitrile
A technology of terephthalonitrile and synthesis method, applied in chemical instruments and methods, ammonia-carboxylic acid reaction preparation, carboxylic acid amide dehydration preparation and other directions, can solve the problems of difficulty in further improvement, great influence, and high production, etc., Achieve the effect of improving yield, reducing oxidation and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
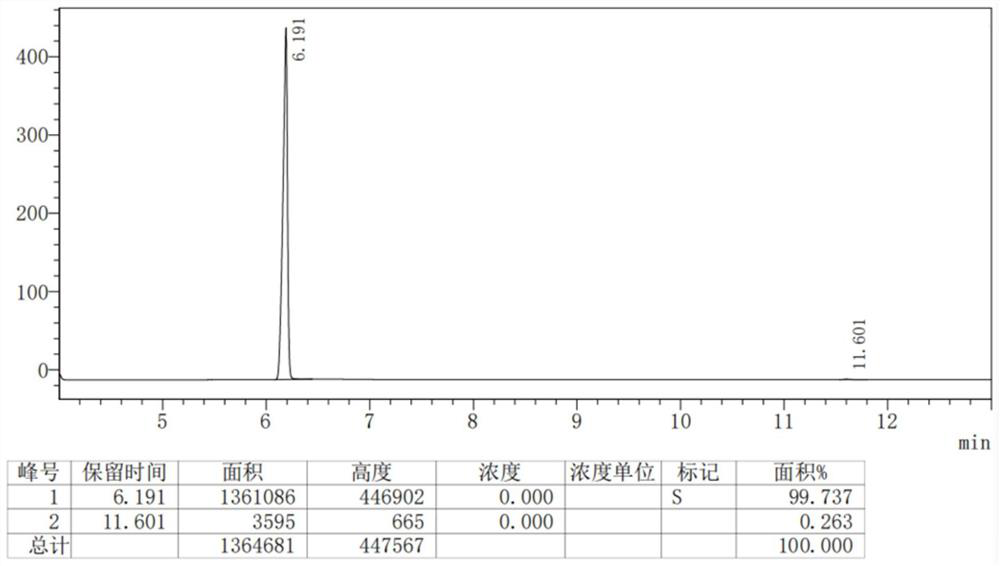
Image
Examples
preparation example Construction
[0056] The present invention also provides the preparation method of the vanadium-chromium-phosphorus catalyst and the vanadium-chromium-phosphorus alkali metal catalyst, the method is that after the vanadium source is reduced, it is mixed with the chromium source, the phosphorus source and other metal sources to form a mixed solution II. On the carrier, the catalyst is obtained by calcining. Described concretely comprises the following steps:
[0057] Step a, dissolving the vanadium source in the reducing liquid, stirring and dissolving to obtain the mixed solution I.
[0058] The vanadium source is selected from pentavalent vanadium compounds, preferably selected from V 2 o 5 Or ammonium metavanadate, more preferably V 2 o 5 . The reducing liquid is oxalic acid aqueous solution, methanol or isobutanol, preferably oxalic acid.
[0059] The molar ratio of the vanadium source to the reducing liquid is 1:(1.1-14), preferably 1:(1.5-10), more preferably 1:(2-6). Wherein, t...
Embodiment 1
[0087] At room temperature, slowly dissolve 12.0 g of vanadium pentoxide in 220 g of oxalic acid aqueous solution containing 60 g, and stir to react. Heat above-mentioned solution to 80 ℃, add 3.0g chromium trioxide again, after it dissolves, add the phosphoric acid solution of the 85% concentration of 28g and 3.5g ammonium orthomolybdate ((NH 4 ) 2 MoO 4 ), stirred for 1 hour.
[0088] The above solution was added to 110 g of silica gel with a particle size of 80-120 mesh. The silica gel was preheated to about 85°C in advance, stirred evenly, left to stand at room temperature for 24 hours, and filtered to obtain a catalyst precursor.
[0089] Place the catalyst precursor in a muffle furnace and gradually raise the temperature to 620°C and keep it warm for 10 hours. After cooling to room temperature, a vanadium-chromium-phosphorus catalyst is obtained, which is bottled for later use. In the vanadium chromium phosphorus catalyst, the molar ratio of each element is V:Cr:P:Mo=...
Embodiment 2
[0091] At room temperature, slowly dissolve 10.0 g of vanadium pentoxide in 200 g of aqueous solution containing 75 g of oxalic acid, and stir to react. Heat the above solution to 80°C, add 6.5g of chromium trioxide and 2.5g of nickel chloride hexahydrate in sequence, after they dissolve, add 2.2g of cesium sulfate, stir and mix, after completely dissolving, add 25g of 85% phosphoric acid solution , add 1.0 g of potassium chloride, and stir for 1 hour.
[0092] The above solution was added to 100 g of silica gel with a particle size of 80-120 mesh. The silica gel was preheated to 85°C in advance, stirred evenly, left standing at 20-30°C for 24 hours, and filtered to obtain a catalyst precursor.
[0093] Take it out and place it in a muffle furnace to gradually raise the temperature to 640° C. and keep it warm for 12 hours. After cooling to room temperature, a vanadium-chromium-phosphorus alkali metal catalyst is obtained, which is bottled for later use. In the vanadium chromi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com