A kind of preparation method and application of manganese oxide composite carbon nitride composite photocatalyst
A technology of manganese tetroxide composite carbon nitride and nanotubes, which can be used in physical/chemical process catalysts, chemical instruments and methods, oxidized water/sewage treatment, etc. The problems of limited oxidation active sites and large photocatalytic electron transition energy threshold can achieve the effect of improving the utilization rate and reaction rate, improving the photocatalytic efficiency and reducing the loading capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
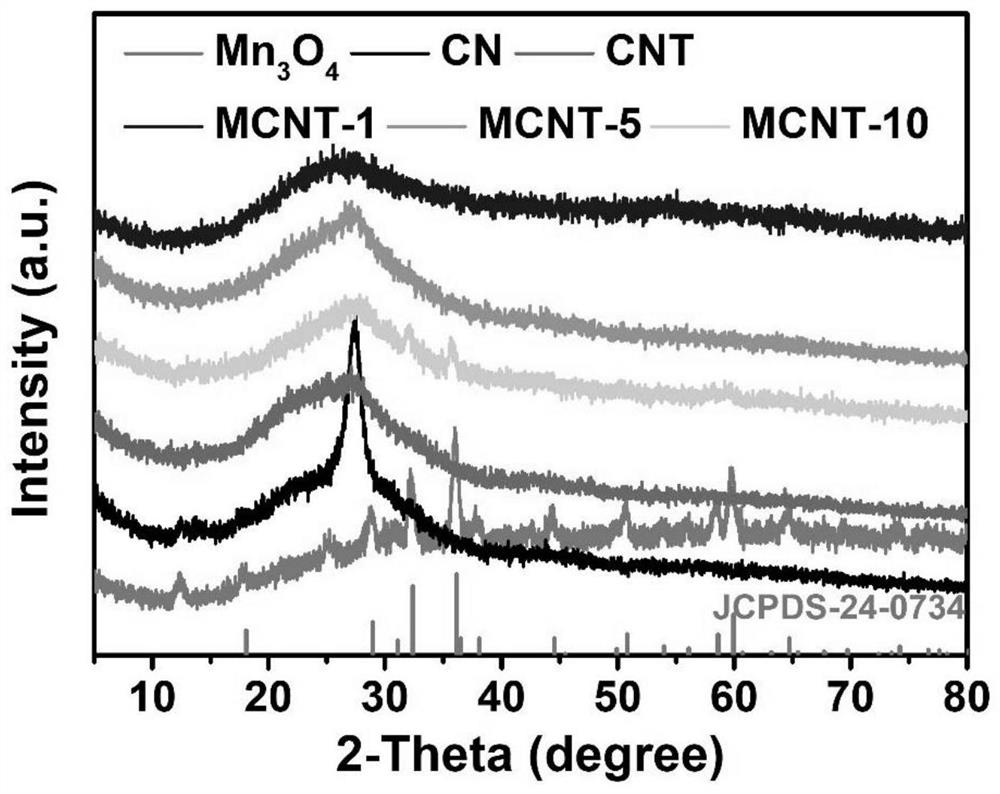
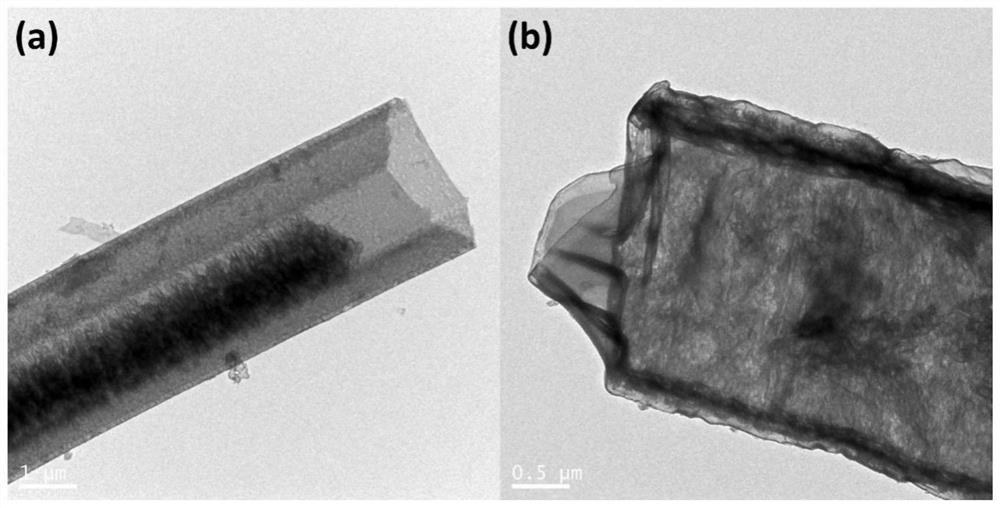
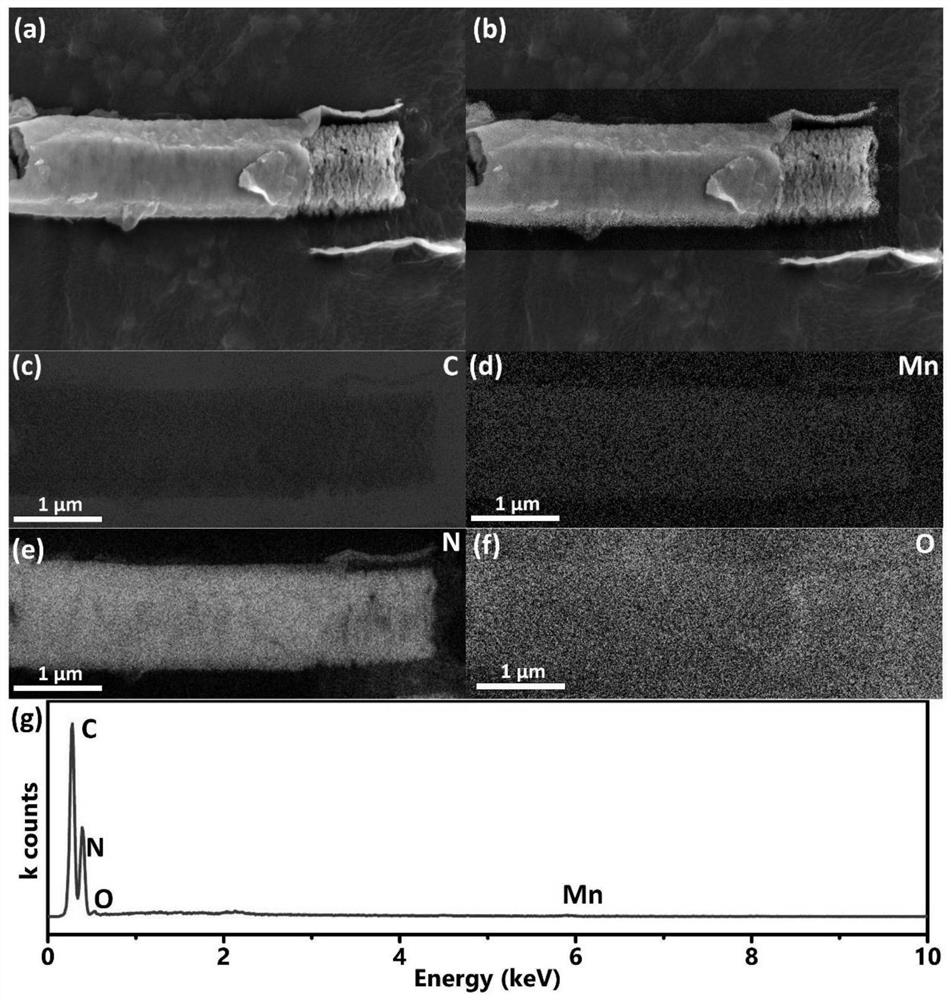
[0033] Preparation of Carbon Nitride Nanotubes (CNTs)
[0034] Weigh 1.0 g of melamine and disperse it into 70 mL of deionized water, heat and stir in an oil bath at 80°C until the melamine is completely dissolved. The obtained transparent solution was transferred to a hydrothermal kettle, and the hydrothermal kettle was heated in an oven at 200° C. for 10 h. After it naturally dropped to room temperature, the obtained sample was washed with water and absolute ethanol three times, and dried to obtain melamine nanorods. The melamine nanorods were placed in a porcelain ark, raised to 550°C at a rate of 2.5°C / min in a muffle furnace and held for 4 hours. After the calcination process, it was naturally cooled to room temperature, the obtained yellow sample was fully ground, washed with water three times, and dried in a vacuum oven at 60°C.
[0035] Loading of trimanganese tetraoxide
[0036] 0.1g CNT, 70mL 1mM Na 2 SO 4 Mix evenly to form a solution, form solution A after ult...
Embodiment 2
[0038] Preparation of Carbon Nitride Nanotubes (CNTs)
[0039] Weigh 1.0 g of melamine and disperse it into 70 mL of deionized water, heat and stir in an oil bath at 80°C until the melamine is completely dissolved. The obtained transparent solution was transferred to a hydrothermal kettle, and the hydrothermal kettle was heated in an oven at 200° C. for 10 h. After it naturally dropped to room temperature, the obtained sample was washed with water and absolute ethanol three times, and dried to obtain melamine nanorods. The melamine nanorods were placed in a porcelain ark, raised to 550°C at a rate of 2.5°C / min in a muffle furnace and held for 4 hours. After the calcination process, it was naturally cooled to room temperature, the obtained yellow sample was fully ground, washed with water three times, and dried in a vacuum oven at 60°C.
[0040] Loading of trimanganese tetraoxide
[0041] 0.1g CNT, 70mL 1mM Na 2 SO 4 Mix evenly to form a solution, form solution A after ult...
Embodiment 3
[0043] Preparation of Carbon Nitride Nanotubes (CNTs)
[0044] Weigh 1.0 g of melamine and disperse it into 70 mL of deionized water, heat and stir in an oil bath at 80°C until the melamine is completely dissolved. The obtained transparent solution was transferred to a hydrothermal kettle, and the hydrothermal kettle was heated in an oven at 200° C. for 10 h. After it naturally dropped to room temperature, the obtained sample was washed with water and absolute ethanol three times, and dried to obtain melamine nanorods. The melamine nanorods were placed in a porcelain ark, raised to 550°C at a rate of 2.5°C / min in a muffle furnace and held for 4 hours. After the calcination process, it was naturally cooled to room temperature, the obtained yellow sample was fully ground, washed with water three times, and dried in a vacuum oven at 60°C.
[0045] Loading of trimanganese tetraoxide
[0046] 0.1g CNT, 70mL 1mM Na 2 SO 4 Mix evenly to form a solution, form solution A after ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com