Inert transition metal element doped iron-based Prussian blue sodium ion battery positive electrode material
A transition metal element, sodium ion battery technology, applied in the direction of battery electrodes, secondary batteries, metal cyanide, etc., can solve the problems of affecting the cycle stability of materials, affecting the electrochemical performance of materials, reducing the Na content of materials, etc., to achieve improved Effects of cycle stability, volume change reduction, and lattice distortion reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 4.5 mmol FeSO 4 ·7H 2 O, 0.5 mmol Zn(CH 3 COO) 2 2H 2 O and 25 mmol Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 50 ml of deionized water to form solution A, 5 mmol Na 4 Fe(CN) 6 10H 2 O and 1 g C 6 h 8 o 6 Dissolve in 50 ml deionized water to form solution B; 1 g polyvinylpyrrolidone PVP and 3 g NaCl are dissolved in deionized water to form solution C; simultaneously add solution A and solution B dropwise to solution C at a rate of 0.167 ml / min During the process, stir and heat while adding dropwise. After the dropwise addition is completed, the solution becomes a white suspension D. After continuing to stir for 12 h, age for 24 h; then use deionized water and Absolute ethanol was centrifuged and washed three times; finally, the dark blue solid was placed in a vacuum oven at 120 o C dried for 24 h to obtain the target zinc-doped Fe-based Prussian blue cathode material, labeled as 10%Zn-FeHCF. The obtained 10% Zn-FeHCF positive electrode material was stirred...
Embodiment 2
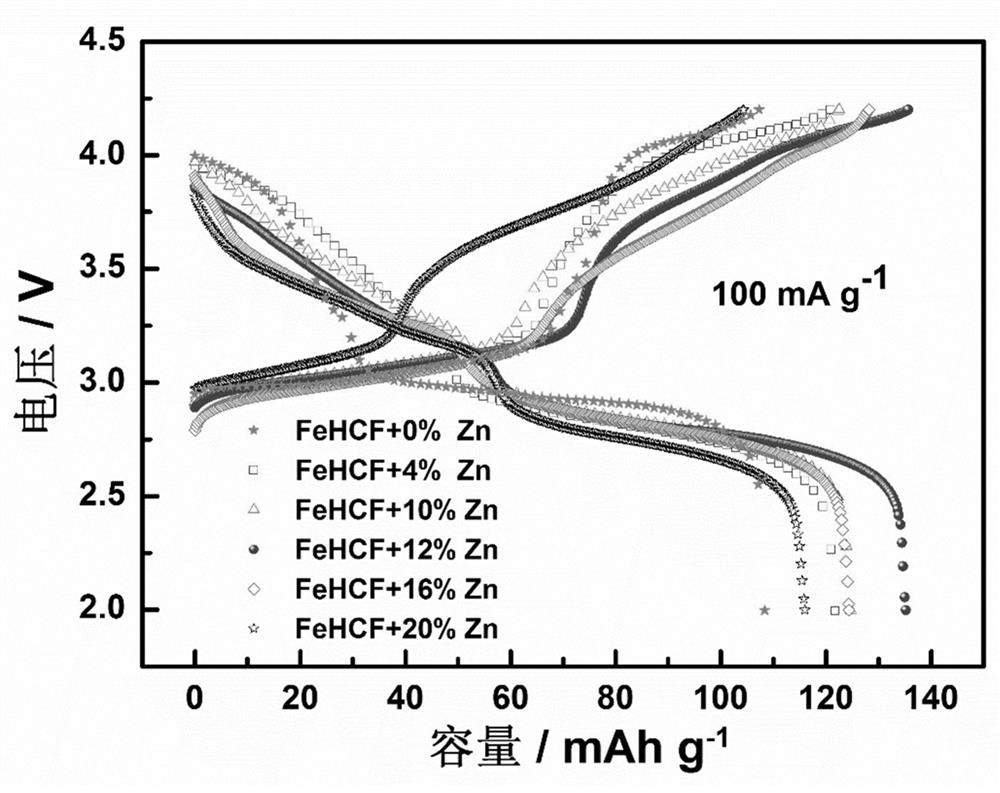
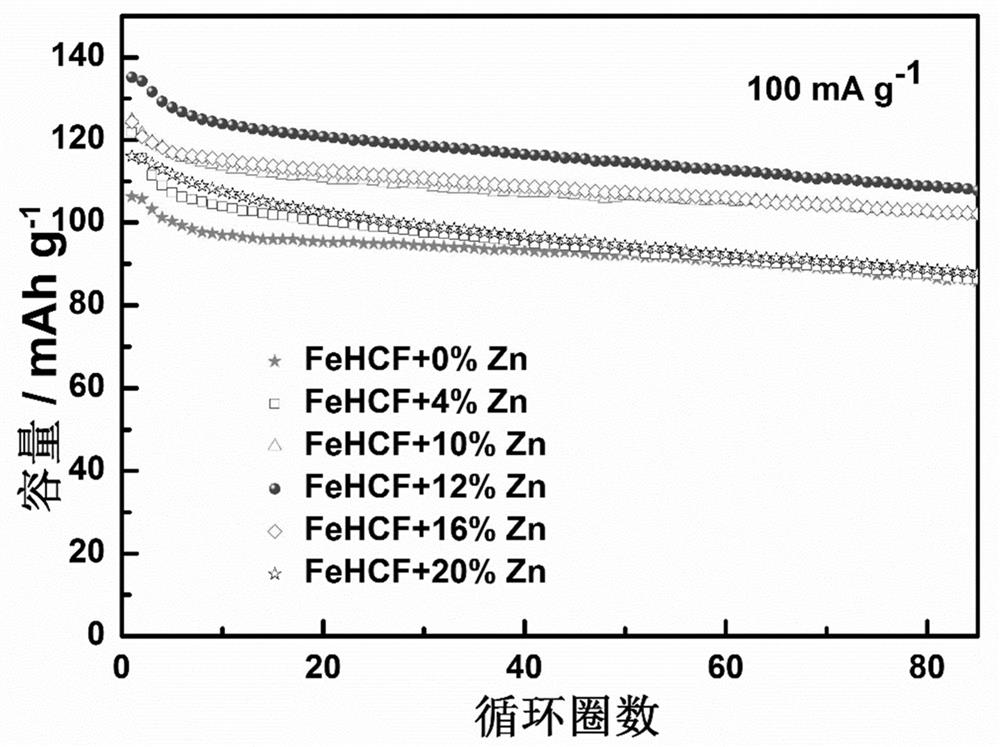
[0032] The preparation steps are the same as in Example 1, only FeSO 4 ·7H 2 O is 4.8 mmol, Zn(CH 3 COO) 2 2H 2 O was 0.2 mmol, and the target zinc-doped iron-based Prussian blue cathode material was prepared, which was labeled as 4%Zn-FeHCF. The obtained 4% Zn-FeHCF positive electrode material was stirred with acetylene black and polyvinylidene fluoride (PVDF) to form a slurry, coated on an aluminum foil, dried, punched and pressed to make a positive electrode material pole piece. Using sodium metal as the counter electrode, Grade GF / D as the separator, 1 M NaClO containing 2 wt.% FEC 4 / (EC+DMC+EMC) (EC:DMC:EMC=1:1:1) is assembled into a battery with electrolyte for constant current charge and discharge test, and the voltage range is between 2.0~4.2 V. 4%Zn-FeHCF cathode material at 100 mA g -1 The initial discharge capacity is only 121.8 mAh g at a high current density -1 , and its specific discharge capacity is only 87.6 mAh g after 80 cycles -1 .
Embodiment 3
[0034] The preparation steps are the same as in Example 1, only FeSO 4 ·7H 2 O is 4.4 mmol, Zn(CH 3 COO) 2 2H 2 O was 0.6 mmol, and the target zinc-doped Fe-based Prussian blue cathode material was prepared, labeled as 12%Zn-FeHCF. The obtained 12% Zn-FeHCF positive electrode material was stirred with acetylene black and polyvinylidene fluoride (PVDF) to form a slurry, coated on an aluminum foil, dried, punched and pressed to make a positive electrode material pole piece. Using sodium metal as the counter electrode, Grade GF / D as the separator, 1 MNaClO containing 2 wt.% FEC 4 / (EC+DMC+EMC) (EC:DMC:EMC=1:1:1) is assembled into a battery with electrolyte for constant current charge and discharge test, and the voltage range is between 2.0~4.2 V. 12%Zn-FeHCF cathode material at 100 mA g -1 The initial discharge capacity at a current density of 135.2 mAh g -1 , and its specific discharge capacity after 80 cycles is 108.8 mAh g -1 .
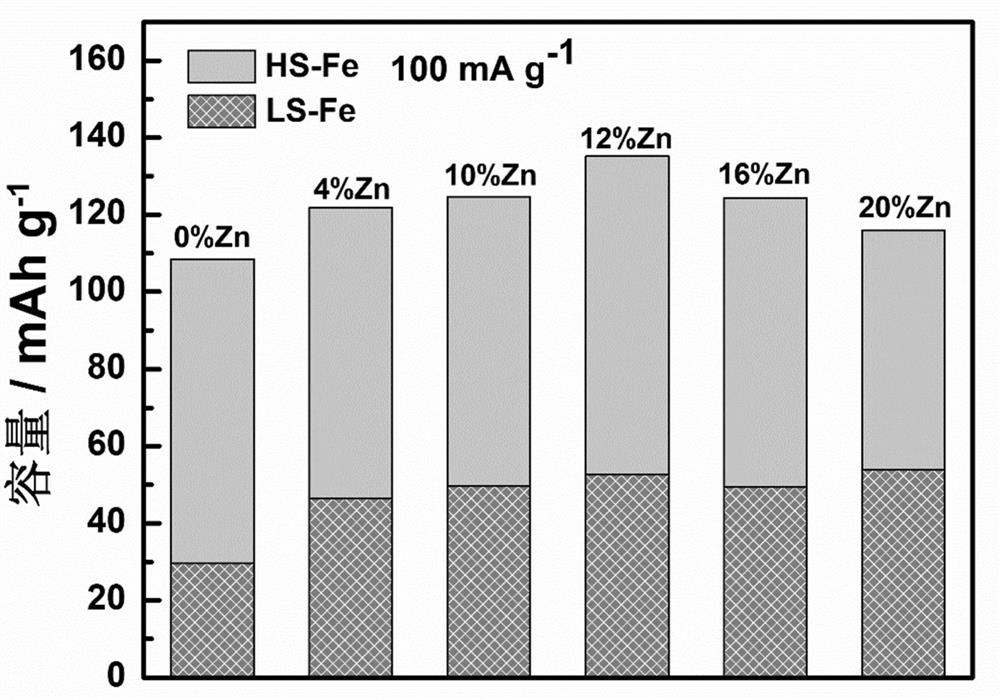
[0035] image 3 It is a comparison ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com