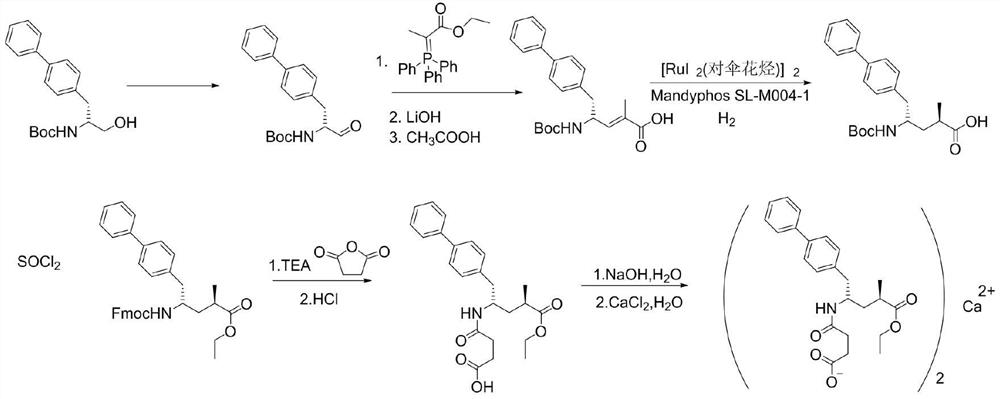
Preparation method of sacubitril intermediate
A technology for sacubitril and intermediates, which is applied in the field of preparation of sacubitril intermediates, which can solve the problems of many impurities and low conversion rate, and achieve the effects of high purity, easy availability of raw materials, and short process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 15.2g of 2-bromopropionic acid and 7.5g of zinc powder into the reaction flask, add toluene (152mL) into the reaction flask, cool down to 0-10°C, and add trimethylchlorosilane solution (32.6mL) dropwise under stirring After dropping, continue stirring for 1 h, TLC [developer: petroleum ether: ethyl acetate = 5:1] detects that the reaction is complete, adds water (150 mL) to the reaction solution, and separates the light yellow clear organic Phase is Compound I solution.
[0050]Add (2R)-1-((1,1'-biphenyl)-4-yl)-3-formylpropan-2-yl-carbamic acid tert-butyl ester (27.1g) to the reaction flask, Add isopropyl acetate (406mL) into the bottle, heat the reaction solution to 10-30°C, add compound I solution to it and stir for 2 hours, TLC [developer: petroleum ether: ethyl acetate = 5:1] to detect the reaction At the end, concentrate under reduced pressure at 35°C to dryness, dissolve in isopropyl acetate (115 mL) with a mass volume ratio of 3 times, and add n-heptane with...
Embodiment 2
[0053] Add 15.2g of 2-bromopropionic acid and 7.5g of zinc powder into the reaction flask, add toluene (230mL) into the reaction flask, cool down to 0-10°C, and add trimethylchlorosilane solution (37.6mL) dropwise under stirring After dropping, continue stirring for 1 h, TLC [developer: petroleum ether: ethyl acetate = 5:1] detects that the reaction is complete, adds water (230 mL) to the reaction solution, and separates the light yellow clear organic Phase is Compound I solution.
[0054] (2R)-1-((1,1'-biphenyl)-4-yl)-3-formylpropan-2-yl-aminofluorenylmethoxycarbonyl ester (37.3 g) was added to the reaction flask, Add isopropyl acetate (670mL) to the reaction flask, heat the reaction solution to 10-30°C, add the compound I solution to it and stir for 2h, TLC [developing solvent: petroleum ether: ethyl acetate = 5:1] After detecting the end of the reaction, concentrate under reduced pressure at 35°C to dryness, dissolve in isopropyl acetate (115mL) with a mass volume ratio of...
Embodiment 3
[0057] Add 15.2g of 2-bromopropionic acid and 7.5g of zinc powder into the reaction flask, add toluene (76mL) into the reaction flask, cool down to 0-10°C, add trimethylchlorosilane solution (30mL) dropwise under stirring, After dropping, continue to stir the reaction for 1 h, TLC [developer: petroleum ether: ethyl acetate = 5:1] detects that the reaction is complete, adds water (76 mL) to the reaction solution, and separates the light yellow clear organic phase. Compound I solution.
[0058] Add (2R)-1-((1,1'-biphenyl)-4-yl)-3-formylpropan-2-yl-carbamic acid tert-butyl ester (27.1g) to the reaction flask, Add isopropyl acetate (325mL) into the bottle, heat the reaction solution to 10-30°C, add compound I solution to it and stir for 2 hours, TLC [developing solvent: petroleum ether: ethyl acetate = 5:1] to detect the reaction At the end, concentrate under reduced pressure at 35°C to dryness, dissolve in isopropyl acetate (115 mL) with a mass volume ratio of 3 times, and add n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com