Local long-acting drug release system for bone disease treatment, and preparation method and application thereof
A technology for the treatment of drugs and bone diseases, applied in the field of local long-acting drug release system for bone disease treatment and its preparation, can solve the problems that have not been fully developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
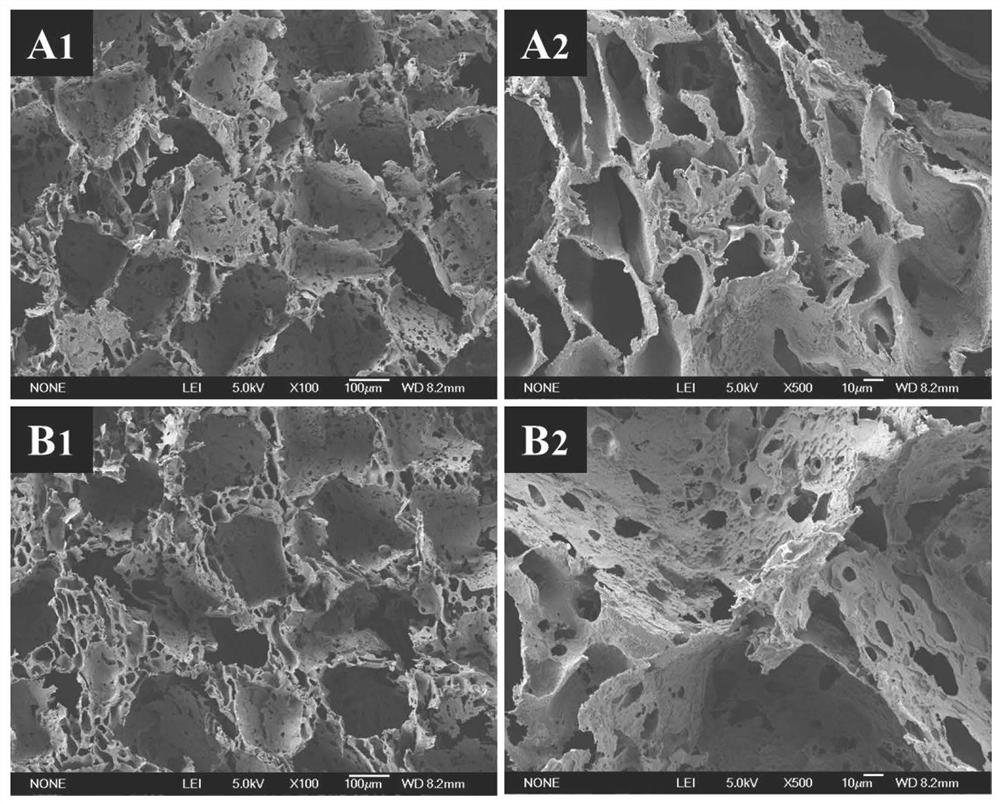
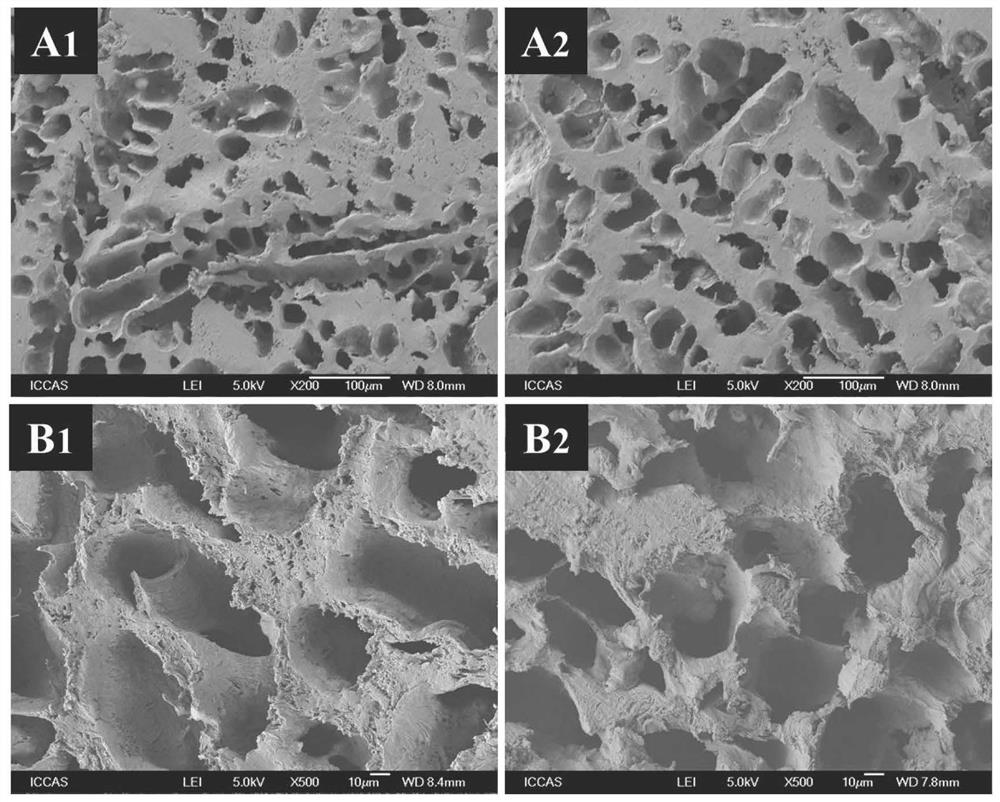
Image
Examples
Embodiment 1
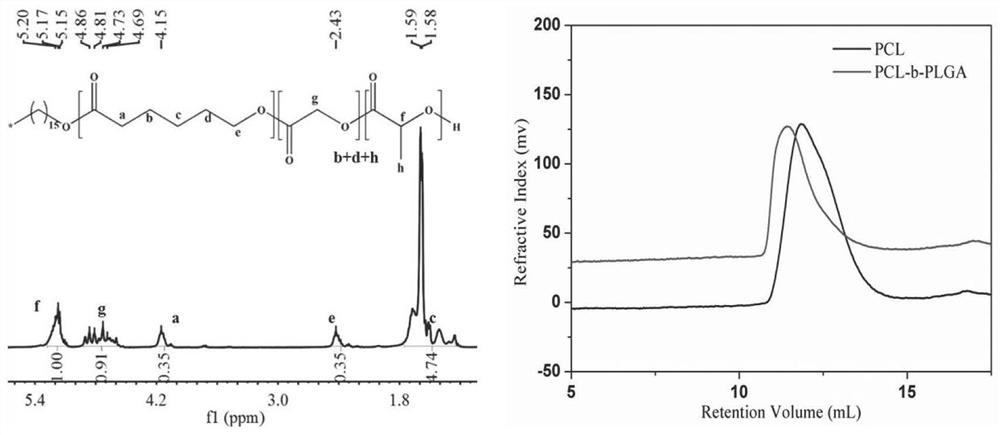
[0063] The preparation of embodiment 1 poly(caprolactone-lactide-glycolide) block polymer
[0064] Weigh 0.0147g of cetyl alcohol and 0.8406g of caprolactone into a dry polymerization tube, mix evenly and place under dry conditions (inert gas protection such as nitrogen, argon or humidity lower than 20% dry air atmosphere or vacuum conditions) , add 0.005g of stannous isooctanoate, and then polymerize at 100°C-200°C for 10-50 hours under vacuum conditions (below 150Pa). The obtained product is purified by dissolution-precipitation method (dissolving the polymer in one or several good solvents such as chloroform, dichloromethane, acetone, tetrahydrofuran, etc., and precipitating in poor solvents such as ethanol, ether, or petroleum ether) After that, the prepolymer PCL (M w =20,000), vacuum-dried and set aside.
[0065] Add 1.53g of prepolymer PCL with a number average molecular weight of 20,000, 6.37g of lactide, and 2.33g of glycolide into a dry polymerization tube, and mix...
Embodiment 2
[0068] The preparation of embodiment 2 poly(caprolactone-lactide-glycolide) block polymer
[0069]Weigh 0.0147g of cetyl alcohol and 0.8406g of caprolactone into a dry polymerization tube, mix evenly and place under dry conditions (inert gas protection such as nitrogen, argon or humidity lower than 20% dry air atmosphere or vacuum conditions) , add 0.005g of stannous isooctanoate, and then polymerize at 100°C-200°C for 10-50 hours under vacuum conditions (below 150Pa). The obtained product is purified by dissolution-precipitation method (dissolving the polymer in one or several good solvents such as chloroform, dichloromethane, acetone, tetrahydrofuran, etc., and precipitating in poor solvents such as ethanol, ether, or petroleum ether) After that, the prepolymer PCL (M w =20,000), vacuum-dried and set aside.
[0070] Add 1.53g of prepolymer PCL with a molecular weight of 20,000, 4.69g of lactide, and 3.78g of glycolide into a dry polymerization tube, mix well and then dry i...
Embodiment 3
[0072] The preparation of embodiment 3 poly(caprolactone-lactide-glycolide) block polymer
[0073] Weigh 0.0147g of cetyl alcohol, 0.8406g of caprolactone, add in the dry polymerization tube, mix well and then in dry conditions (inert gas protection such as nitrogen, argon or humidity is lower than 20% dry air atmosphere or vacuum conditions) 0.005g stannous isooctanoate was added, and then polymerized at 100°C-200°C for 10-50 hours under vacuum conditions (below 150Pa). The obtained product is purified by dissolution-precipitation method (dissolving the polymer in one or several good solvents such as chloroform, dichloromethane, acetone, tetrahydrofuran, etc., and precipitating in poor solvents such as ethanol, ether, or petroleum ether) After that, the prepolymer PCL (M w =20,000), vacuum-dried and set aside.
[0074] Add the prepolymer PCL with a weight-average molecular weight of 20,000, 5.14g lactide, and 1.78g glycolide into the dry polymerization tube, and mix them un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com