Cathode materials and applications for improving the interfacial stability of sulfide electrolytes
A sulfide electrolyte and positive electrode material technology, applied in the field of materials, can solve the problems of unstudied material optimization and modification of the performance of all-solid-state sulfide batteries, so as to reduce the electrochemical side reactions at the interface, increase the capacity, and reduce the interface impedance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
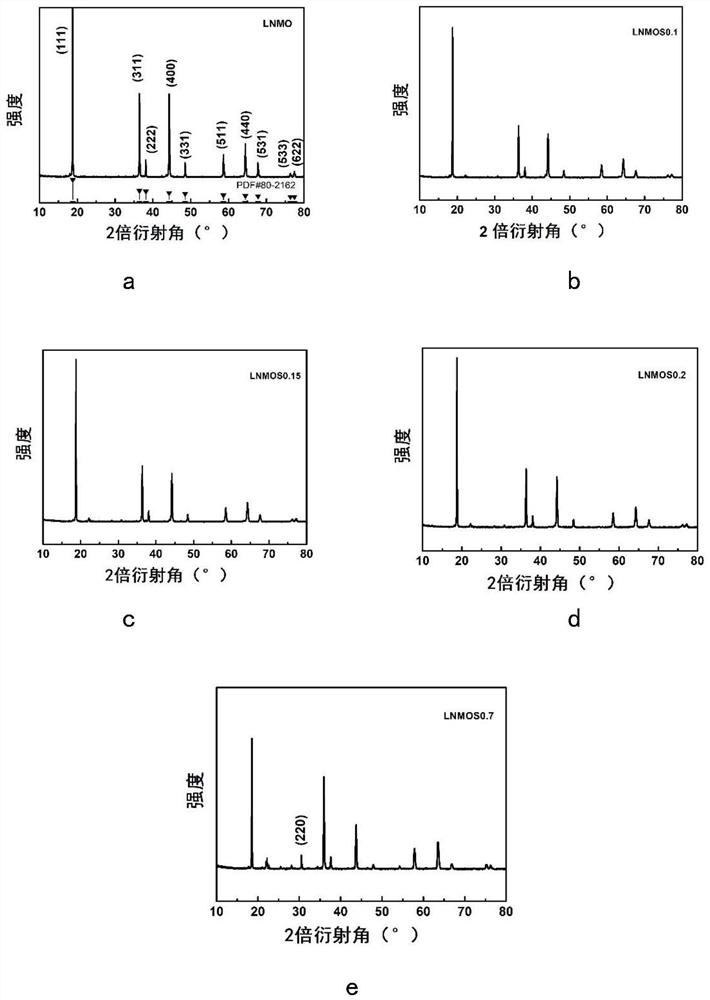
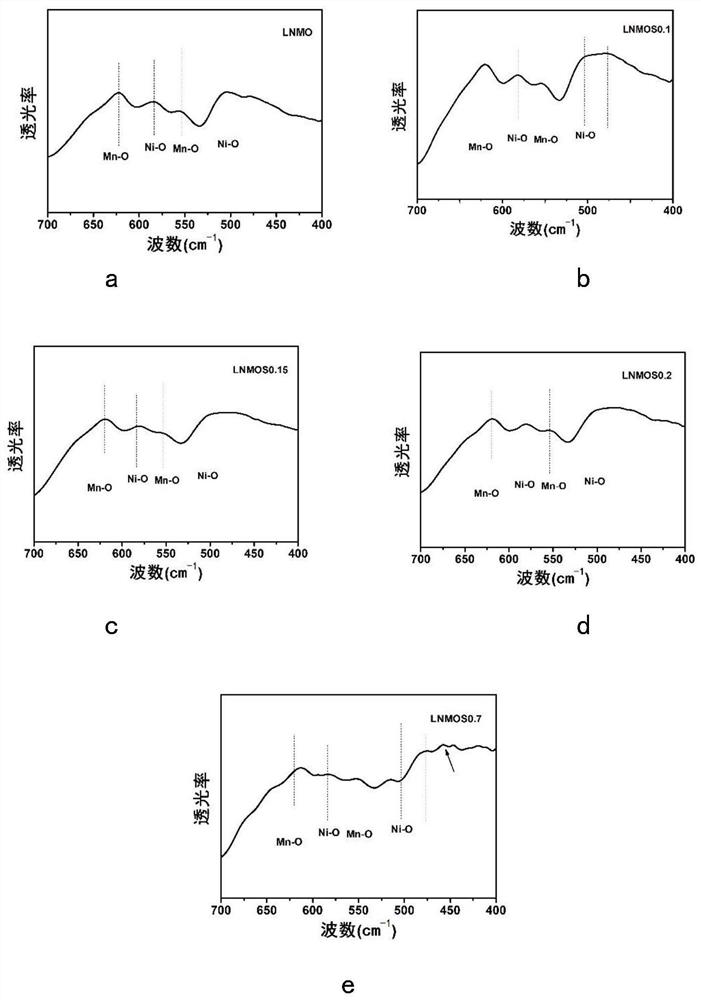
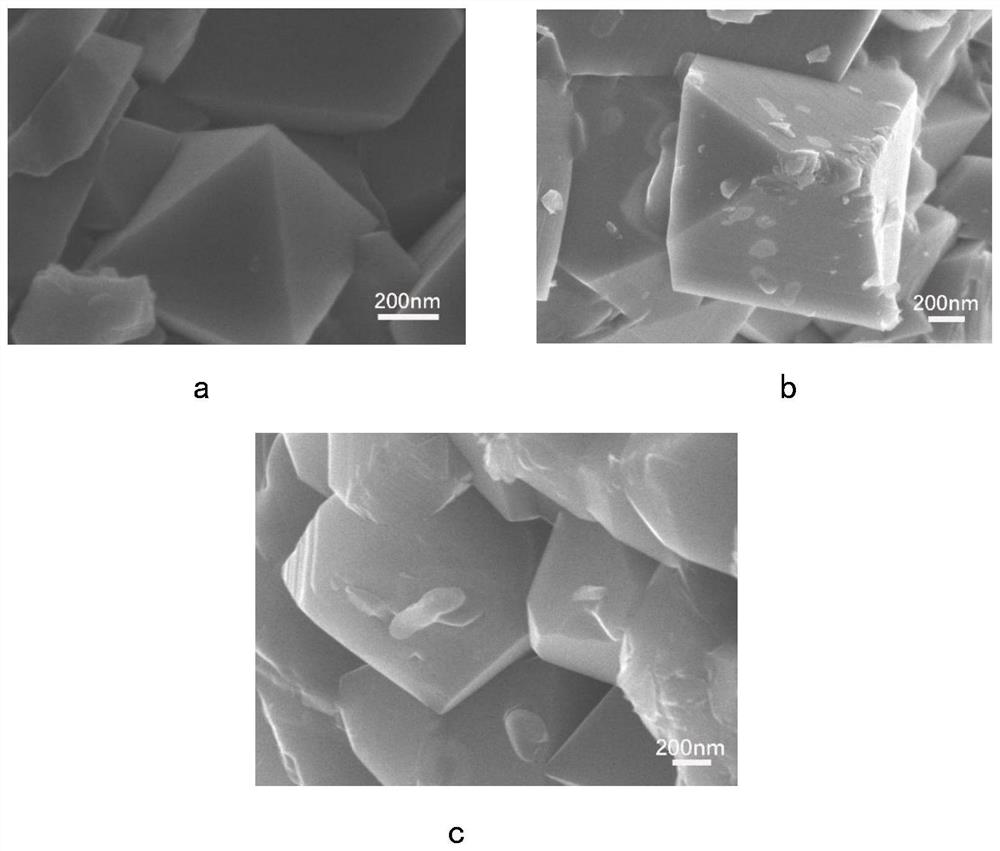
[0056] This embodiment is used to compare raw material LiNi 0.5 mn 1.5 o 4 , LiNi protected by the present invention 0.5 mn 1.5 S x o 4-x (x=0.1,0.15,0.2), and excessive doping of S element LiNi beyond the scope of the present invention 0.5 mn 1.5 S x o 4-x (x=0.7) characteristics of the positive electrode material.
[0057] First illustrate the test equipment and test conditions of the present embodiment:
[0058]X-ray diffraction measurements were performed on a Bruker AXSD8 laser with copper Kα radiation of λ = 1.54178 in the range 10° ≤ 2θ ≤ 80°. Scanning electron microscopy tests were performed using a Hitachi S4800 scanning electron microscope, and elemental surface scans of the test surfaces were performed using energy dispersive X-ray spectroscopy (EDX). Fourier Transform Infrared Spectroscopy (FTIR) tests were performed using a Nicolette 8700 infrared spectrometer. The functional groups of all samples were tested using Fourier transform infrared spectrosco...
Embodiment 2
[0078] This embodiment provides the positive electrode material LiNi with cation doping on the basis of the LNMOS0.15 of the above-mentioned embodiment 1. 0.415 mn 1.245 Cu 0.05 Mg 0.02 Y 0.01 B 0.01 Fe 0.1 S 0.15 o 3.85 .
[0079] This embodiment selects nickel-manganese precursor Ni 0.25 mn 0.75 (OH) 2 , the lithium source is Li 2 CO 3 , the sulfur source is sulfur powder.
[0080] Put the nickel-manganese precursor, an excess of 5% lithium source, sulfur powder, copper oxide, magnesium oxide, iron oxide, boric acid, and yttrium oxide in a mortar and grind for 2 hours according to the required molar ratio, so that the lithium source powder and the sulfur source The powder is uniformly mixed with the nickel-manganese precursor to obtain a mixed material. During batch preparation, the material can also be placed in a ball mill tank with a rotating speed of 230-400 rpm, a mixing time of 2-6 hours, and a material filling efficiency of 55% in the equipment to obtain...
Embodiment 3
[0089] This embodiment provides the positive electrode material LiNi with cation doping on the basis of the LNMOS0.15 of the above-mentioned embodiment 1. 0.45 mn 1.35 Al 0.06 Fe 0.1 Ti 0.03 Nb 0.01 S 0.15 o 3.85 .
[0090] This embodiment selects nickel-manganese precursor Ni 0.25 mn 0.75 (OH) 2 , the lithium source is Li 2 CO 3 , the sulfur source is sulfur powder.
[0091] Put the nickel-manganese precursor, an excess of 5% lithium source, sulfur powder, niobium pentoxide, iron oxide, titanium oxide, boric acid, and aluminum oxide in a mortar and grind for 2 hours according to the required molar ratio to make the lithium source powder, The sulfur source powder is uniformly mixed with the nickel-manganese precursor to obtain a mixed material. During batch preparation, the material can also be placed in a ball mill tank with a rotating speed of 230-400 rpm, a mixing time of 2-6 hours, and a material filling efficiency of 55% in the equipment to obtain a mixed mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com