Composition containing sophora flavescens extract for pulmonary administration as well as preparation method and application of composition
A technology for pulmonary administration and extract, applied in the field of compositions containing Sophora flavescens extract and its preparation, can solve the problems of poor drug sedimentation and retention effect, poor drug stability, etc., and achieves improved stability and bioavailability , Improve the effect of sedimentation and retention, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
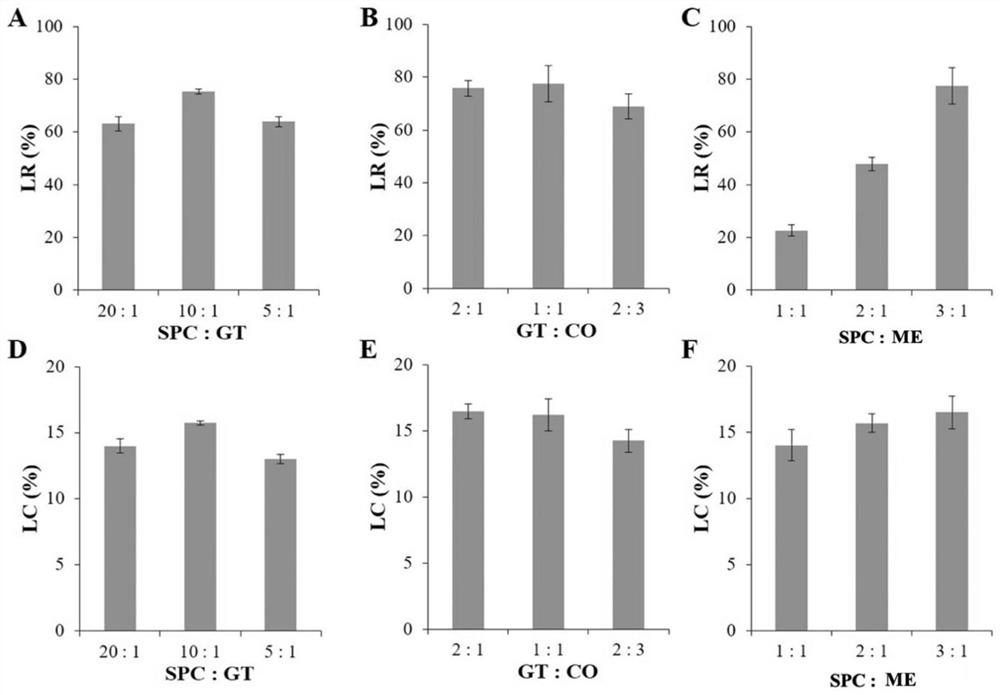
[0042] Weigh 120 mg of soybean lecithin, 12 mg of cholesterol oleate, 6 mg of stearylamine, 12 mg of glyceryl trioleate, and 6 mg of polyethylene glycol monostearate in eggplant-shaped bottles, and add appropriate amount of dichloro Methane was vortexed to dissolve it completely; the organic solvent was removed by rotary evaporation under reduced pressure until the solution was about 0.5 mL. Add 0.5 mL of dichloromethane and 40 mg of oxymatrine, vortex to mix, and continue rotary evaporation for 20 min until a dry and uniform lipid film is formed on the bottle wall. Take out the eggplant-shaped bottle, add 4 mL of 0.01 mmol / L PBS solution to wash the membrane, and hydrate at 25°C for 1 hour to form a liposome primary emulsion. Sonicate the hydrated emulsion in a water bath for 1 min to obtain the Lip / ME solution. Incubate the Lip / ME solution with an equal volume of 40-100KDa hyaluronic acid solution (20mg / mL) at 25°C for 30 min to obtain the Lip / ME@ HA suspension.
[0043] F...
Embodiment 2
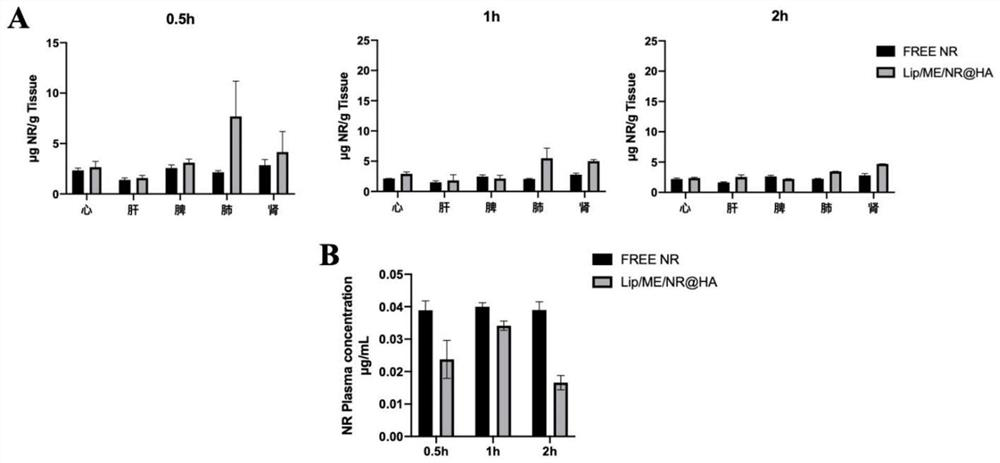
[0045] Example 2 Experimental study on tissue distribution in mice
[0046] The results of the in vivo tissue distribution study showed that the Lip / ME@HA obtained in Example 1 significantly improved the stability of Sophora flavescens extract in mice, prolonged the residence time of the drug in the lungs, and improved the bioavailability of the drug.
[0047] Experimental method: Nile red (NR) was loaded into Lip / ME@HA to form Lip / ME / NR@HA. 36 mice were divided into 2 groups with 18 mice in each group. After being anesthetized with urethane, the free Nile red solution (Free-NR) and Lip / ME / NR@HA were administered with a pulmonary administration quantitative nebulizer, respectively, with a dosage of 0.75 mg / kg. At 0.5, 1, and 2 hours after administration, 0.5 mL of blood was collected from the abdominal aorta and placed in a heparinized centrifuge tube; dissected, and the heart, liver, spleen, lung, and kidney were collected. Whole blood was centrifuged at 4°C, 6000r / min for ...
Embodiment 3
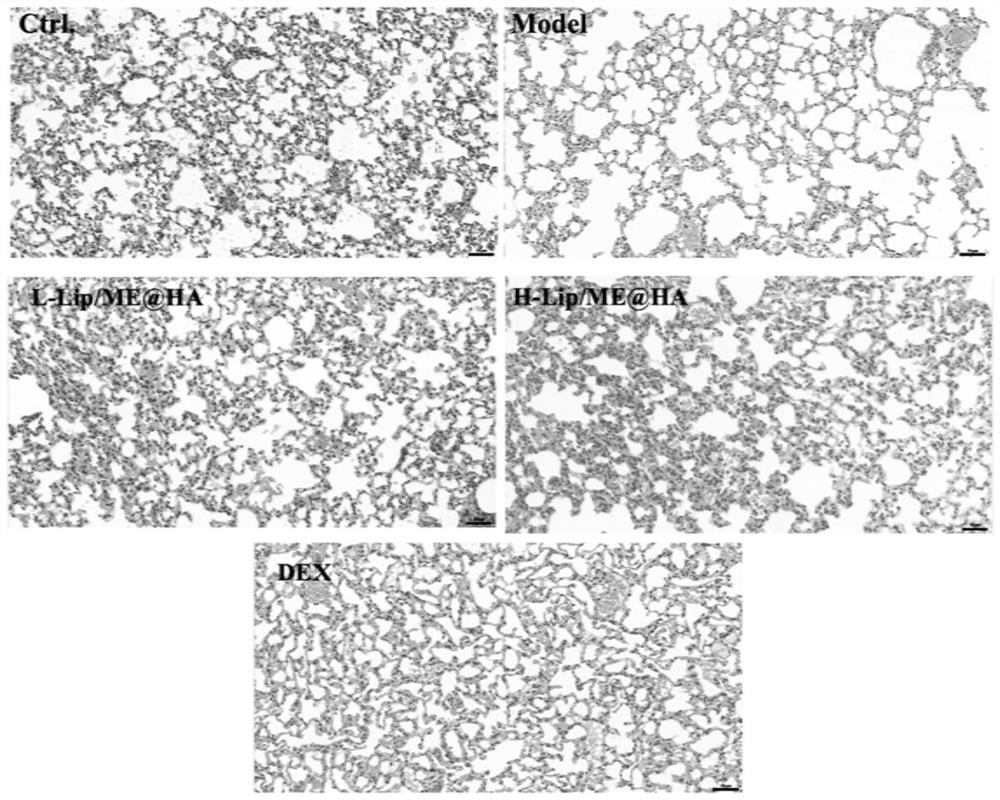
[0049] Example 3 Investigation of the therapeutic effect of Lip / ME@HA preparation on emphysema mice
[0050] The product of implementation example 1 is carried out the experimental study of drug efficacy in animals.
[0051] Preparation group: Lip / ME@HA group
[0052] Control group: normal group, model group, positive drug dexamethasone group
[0053] experimental method
[0054] SPF grade C57BL / 6 mice were randomly divided into 5 groups, normal group (Ctrl), model group (Model), low-dose Lip / ME@HA group (L-Lip / ME@HA), high-dose Lip / ME@ HA group (H-Lip / ME@HA) and positive control dexamethasone group (DEX). After anesthetized by intraperitoneal injection of urethane, 100 μL of porcine pancreatic elastin was injected into the airway on the 1st, 7th, 14th, 21st and 28th day using a pulmonary administration quantitative nebulizer. The Lip / ME@HA low-dose and high-dose groups were given Lip / ME@HA every other day using a pulmonary delivery quantitative nebulizer starting from the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com