Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and lithium hexafluorophosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as difficulty in meeting industrialization needs, difficult industrial-scale production, and difficult reaction control, and achieve high product yields , The effect of reducing production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
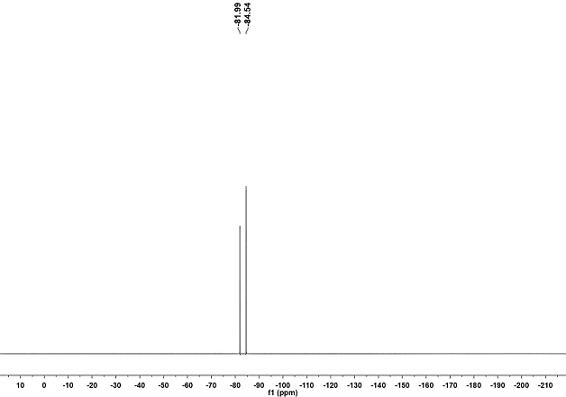
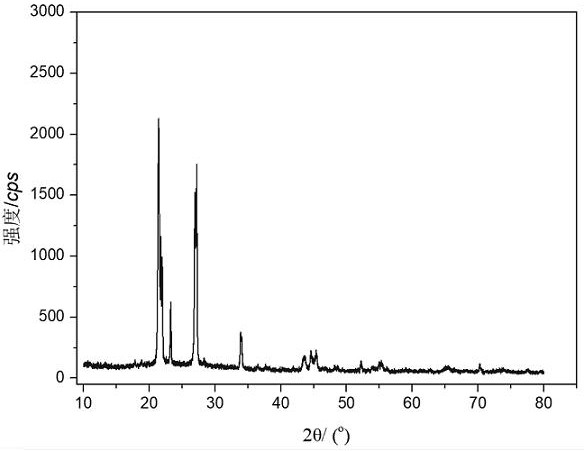
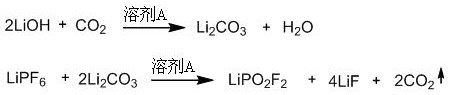
[0023] A method for preparing lithium difluorophosphate, the method refers to sequentially adding solvent A, lithium hexafluorophosphate, and lithium hydroxide into a container, and reacting in a carbon dioxide atmosphere at 25-80°C and 1-40 atm for 1-12 hours to obtain the reaction liquid; the reaction solution was filtered and vacuum-dried to constant weight to obtain a white solid containing lithium difluorophosphate and lithium fluoride. Lithium chloride precipitate and filtrate, the filtrate is lithium difluorophosphate solution. The filtrate was distilled to remove the solvent, and then vacuum-dried at 40-60°C to constant weight to obtain pure white solid anhydrous lithium difluorophosphate.
[0024] Wherein: the molar ratio of lithium hexafluorophosphate and lithium hydroxide is 1: 4~5. The ratio of solvent A to lithium hexafluorophosphate is 1L: 0.2~0.5 mol.
[0025] Solvent A refers to at least one of dimethyl carbonate, diethyl carbonate, ethyl methyl carbonate, me...
Embodiment 1
[0027] Embodiment 1 A kind of preparation method of lithium difluorophosphate, this method refers to:
[0028] Weigh 100 mmol of lithium hexafluorophosphate and 400 mmol of lithium hydroxide, add 300 mL of diethyl carbonate to the reaction bottle, replace the whole system with carbon dioxide atmosphere and keep the reaction pressure at 1 atm, react at 60°C for 5 hours, stop reaction. The reaction solution was filtered and vacuum-dried at 60°C to constant weight to obtain a white solid containing lithium difluorophosphate and lithium fluoride; then the white solid was poured into 80 mL of ethylene glycol dimethyl ether, and the insoluble matter was lithium fluoride. The filter cake after filtration is lithium fluoride, and the recyclable filtrate is lithium difluorophosphate solution; after distilling off the solvent, the white solid is vacuum-dried at 40°C, which is the pure product of anhydrous lithium difluorophosphate, with a yield of 91% and a purity of 99.8%. The above-...
Embodiment 2
[0031] Embodiment 2 A kind of preparation method of lithium difluorophosphate, this method refers to:
[0032] Weigh 100 mmol of lithium hexafluorophosphate and 500 mmol of lithium hydroxide, add 200 mL of diethyl carbonate to the reaction bottle, replace the whole system with carbon dioxide atmosphere and keep the reaction pressure at 1 atm, react at 25°C for 12 hours, stop reaction. The reaction solution was filtered and vacuum-dried at 60°C to constant weight to obtain a white solid containing lithium difluorophosphate and lithium fluoride; then the white solid was poured into 80 mL of ethylene glycol dimethyl ether, and the insoluble matter was lithium fluoride. The filter cake after filtration is lithium fluoride, and the recyclable filtrate is lithium difluorophosphate solution; after distilling off the solvent, the white solid is vacuum-dried at 40°C, which is the pure product of anhydrous lithium difluorophosphate, with a yield of 83% and a purity of 99.8%. The above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com