A kind of preparation method of ferrocyanide and its application in flow battery
A technology of ferrocyanide and liquid flow batteries, which is applied in the direction of ferricyanide, metal cyanide, fuel cells, etc., can solve the problems of difficulty in improving product purity, complex synthesis process, lack of research system, etc., and achieve mild reaction conditions, The effect of high reaction yield and great flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
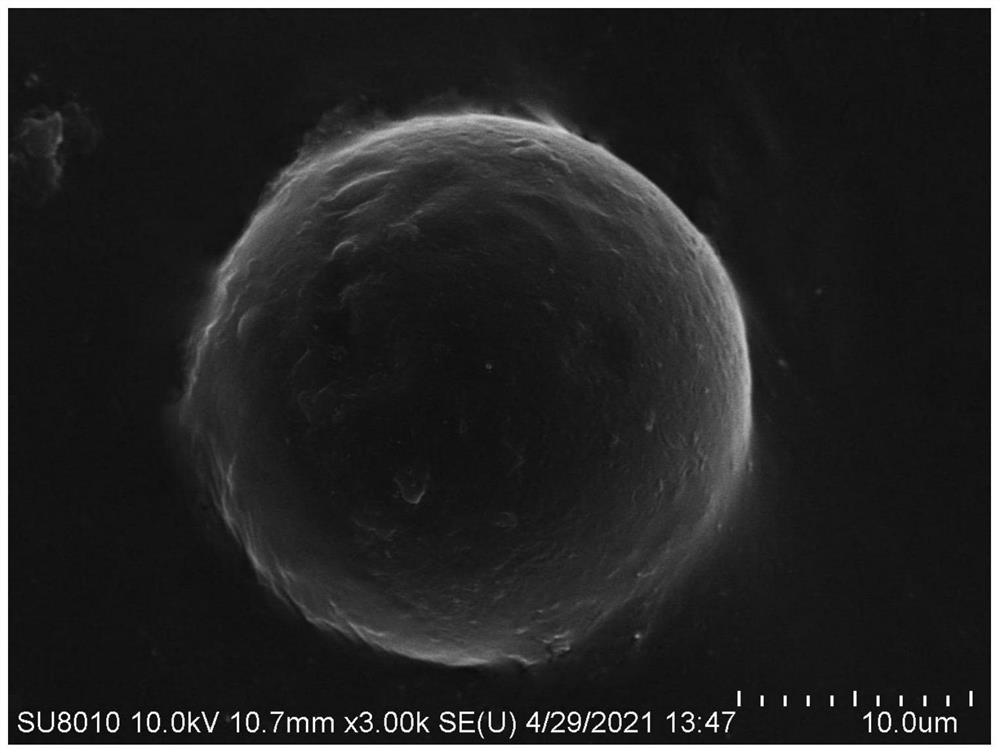
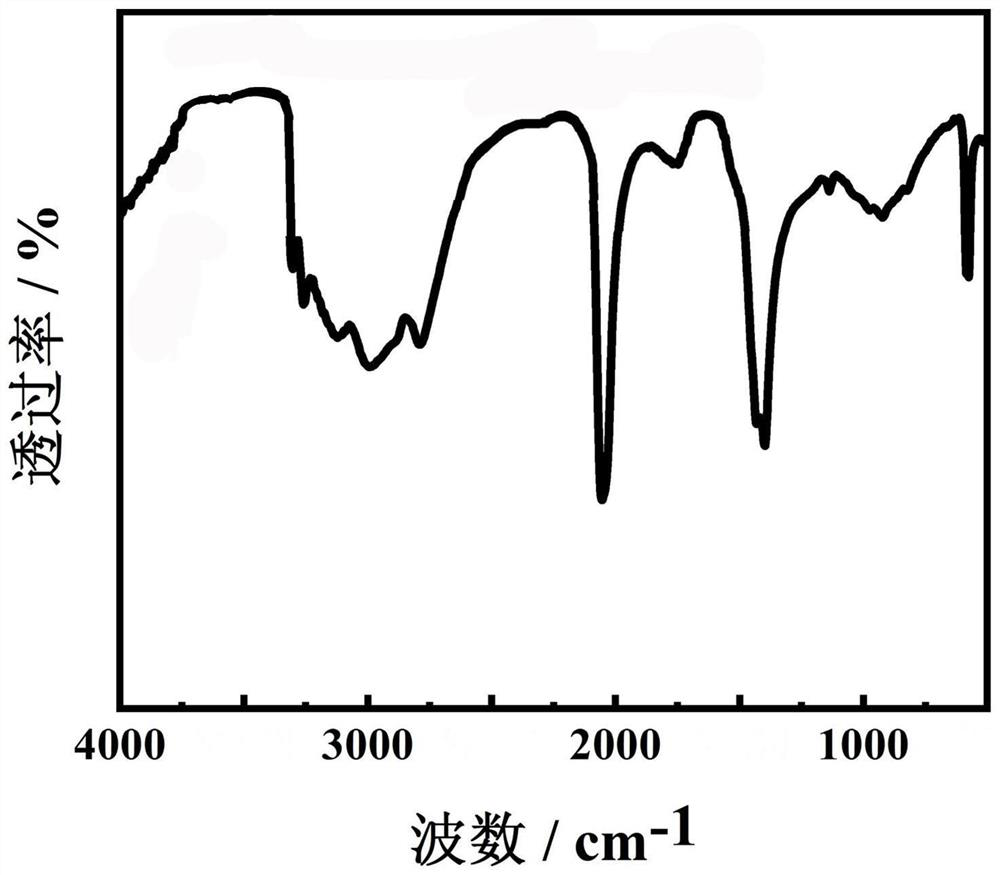
Image
Examples
Embodiment 1
[0050] Cation exchange resin pretreatment:
[0051] (1) A 100g cation exchange resin was weighed in the beaker, soaked in anhydrous ethanol for 24 hours at room temperature.
[0052] (2) Anhydrous ethanol was filtered off, and then the ion exchange resin was allowed to immerse the cation exchange resin for 24 hours with a deionized water chamber.
[0053] (3) Exchange the pre-treated cation exchange resin pillar.
[0054] Preparation steps of ferridanide:
[0055] (1) Tissue 67.6 ml of nitric acid in a dry beaker with a tissue, and then slowly add 250 ml of capacity bottle after dilution with deionized water to set to 250 mL.
[0056] (2) Weigh 62.5 g of potassium hydroxide in a dry beaker, and then slowly add 250 ml of volumetric flask after dilution with deionized water, and set to 250 mL.
[0057] (3) Weigh 13.2 g ammonium chloride in a dry beaker, add 250 ml of capacity bottle after dilution with deionized water, and set to 250 ml
[0058] (4) Configure 200 ml of ferrocyanide ...
Embodiment 2
[0068] Cation exchange resin pretreatment:
[0069] (1) A 100g cation exchange resin was weighed in the beaker, soaked in anhydrous ethanol for 24 hours at room temperature.
[0070] (2) Anhydrous ethanol was filtered off, and then the ion exchange resin was allowed to immerse the cation exchange resin for 24 hours with a deionized water chamber.
[0071] (3) Exchange the pre-treated cation exchange resin pillar.
[0072] Preparation steps of ferridanide:
[0073] (1) Tissue 39.1 ml of sulfuric acid in a drying beaker was used to add 250 ml of capacity bottle after dilution with deionized water, and set to 250 mL.
[0074] (2) Weigh 13.2 g of sodium hydroxide in a dry beaker, slowly add 250 ml of capacity bottle after dilution with deionized water, and set to 250 ml
[0075] (3) Weigh 8.9ml of ammonia water into the dry beaker, slowly add 250 ml of capacity bottle after dilution with deionized water, and set to 250 ml
[0076] (4) Configure 200 ml of ferrocyanide saturation soluti...
Embodiment 3
[0086] Cation exchange resin pretreatment:
[0087] (1) A 100g cation exchange resin was weighed in the beaker, soaked in anhydrous ethanol for 24 hours at room temperature.
[0088] (2) Anhydrous ethanol was filtered off, and then the ion exchange resin was allowed to immerse the cation exchange resin for 24 hours with a deionized water chamber.
[0089] (3) Exchange the pre-treated cation exchange resin pillar.
[0090] Preparation steps of ferridanide:
[0091] (1) Weighing 3.73 ml of nitric acid in a dry beaker with a tissue, a 250 ml capacity bottle was slowly added after dilution with deionized water, and was allowed to 250 mL.
[0092] (2) Weigh 27.8 g of potassium hydroxide in a dry beaker, slowly add 250 ml of volumetric flask after dilution with deionized water, and set to 250ml
[0093] (3) Tissue 27.8 g of lithium hydroxide in the drying beaker, slowly add 250 ml of capacity bottle after dilution with deionized water, and set to 250 ml
[0094] (4) Configure 200 ml of fe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com