Choline chloride eutectic solvent as well as preparation method and application thereof
A low eutectic solvent, choline chloride technology, applied in the production of bulk chemicals, etc., can solve the problems of single type of hydrogen bond acceptor, limited application scope, difficult to meet production needs, etc., and achieves polymerization degree and thermal stability. Small, improved accessibility, low environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of choline chloride deep eutectic solvent
[0035] In the present embodiment, the choline chloride deep eutectic solvent is prepared as a hydrogen bond donor with alcohols, and the alcohols are any one of ethylene glycol, glycerol, propylene glycol, sorbitol, xylitol, and ethyl Glycol is example, and preparation obtains ChCl / ethylene glycol, and concrete steps are as follows:
[0036] Firstly, the raw materials are dried, especially ChCl should be dried in an oven for 4-6 hours before the experiment, and then choline chloride and ethylene glycol are evenly mixed in a beaker (the molar ratio is 1:2), at 100 ℃ Stir continuously until a uniform transparent liquid is formed, then take it out, cool to room temperature, and finally store the prepared DES in a vacuum desiccator for future use.
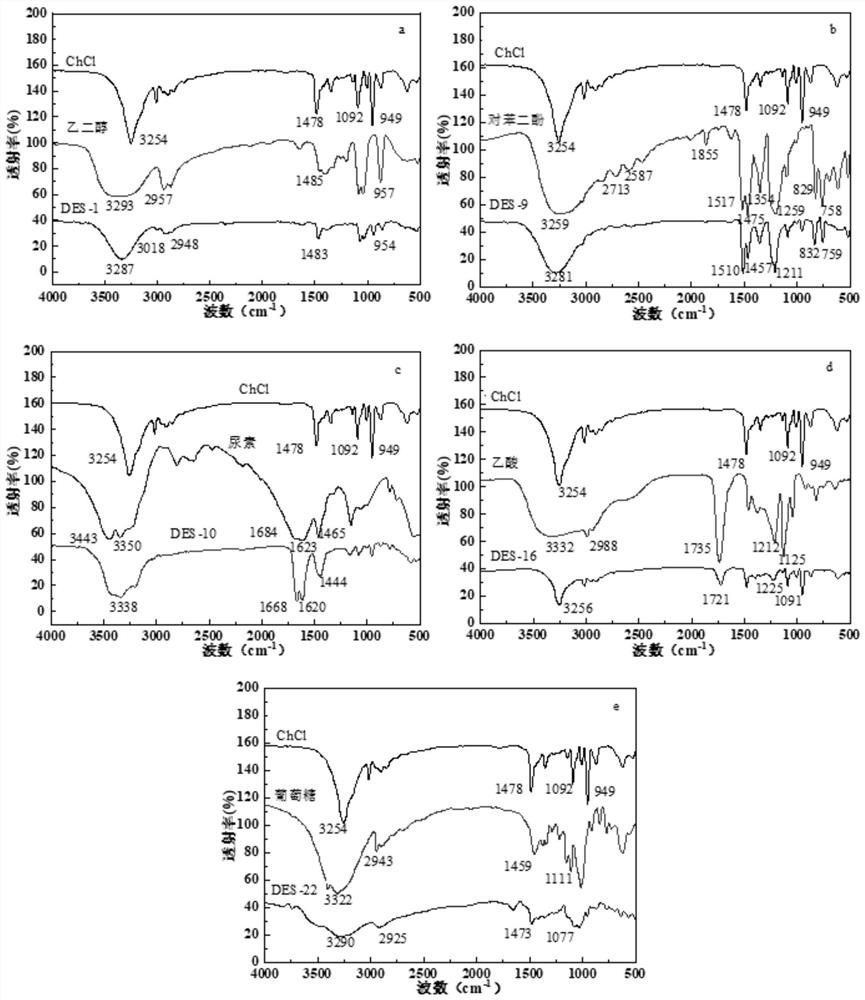
[0037] figure 1 (a) is the infrared spectrum of ChCl / ethylene glycol. from figure 1 (a) It can be seen that ethylene glycol at 3293cm -1 The absorpti...
Embodiment 2
[0038] Embodiment 2: the preparation of choline chloride deep eutectic solvent
[0039]In the present embodiment, the choline chloride deep eutectic solvent is prepared with phenols as hydrogen bond donors, and the phenols are any one of phenol, p-cresol, resorcinol, hydroquinone, etc., Taking hydroquinone as an example to prepare ChCl / hydroquinone, the specific preparation steps are basically the same as in Example 1, except for the following difference: the hydrogen bond donor is hydroquinone.
[0040] figure 1 (b) is the infrared spectrum of ChCl / hydroquinone, from figure 1 In (b), it can be seen that hydroquinone at 3281cm -1 The absorption band broadens and moves down to 3255 cm -1 ; The characteristic spectrum of hydroquinone asC=C and δCH are from (1517cm -1 、1475 cm -1 ) and (832 cm -1 、759 cm -1 ) down to (1510 cm -1 、1457 cm -1 ) and (829 cm -1 、758 cm -1 ), all indicate that there is a hydrogen bond between hydroquinone and choline chloride.
Embodiment 3
[0041] Embodiment 3: the preparation of choline chloride deep eutectic solvent
[0042] In the present embodiment, the choline chloride deep eutectic solvent is prepared with amines as hydrogen bond donors, and the amines are any one of urea, thiourea, acetamide, ethanolamine, triethanolamine, etc., with urea as For example, to prepare ChCl / urea, the specific preparation steps are basically the same as in Example 1, except for the following difference: the hydrogen bond donor is urea.
[0043] figure 1 (c) is the infrared spectrum of ChCl / urea, from figure 1 (c) It can be seen that ChCl / urea at 3443cm -1 and 3350cm -1 Compared with the hydroxyl peak of urea, the absorption band of urea is broadened, and the sNH of urea 2 and δNH 2 The characteristic peak bands are from 1684cm -1 and 1623 cm -1 Move down to 1668 cm -1 and 1620 cm -1 , indicating that there is hydrogen bonding between the two components in ChCl / urea.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com