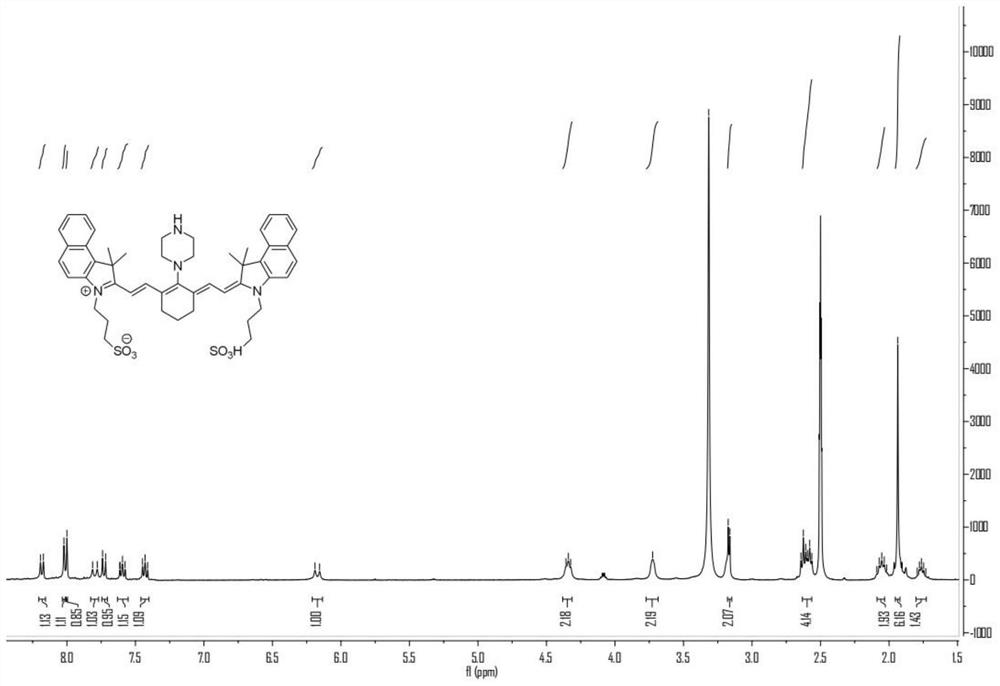
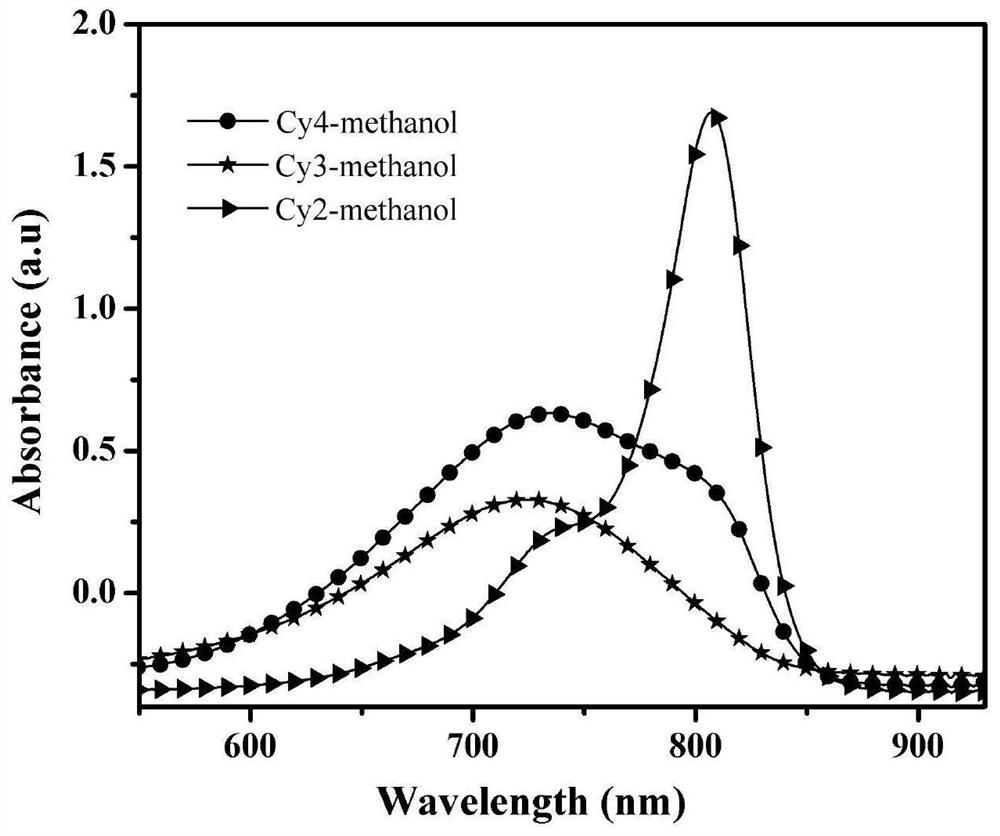
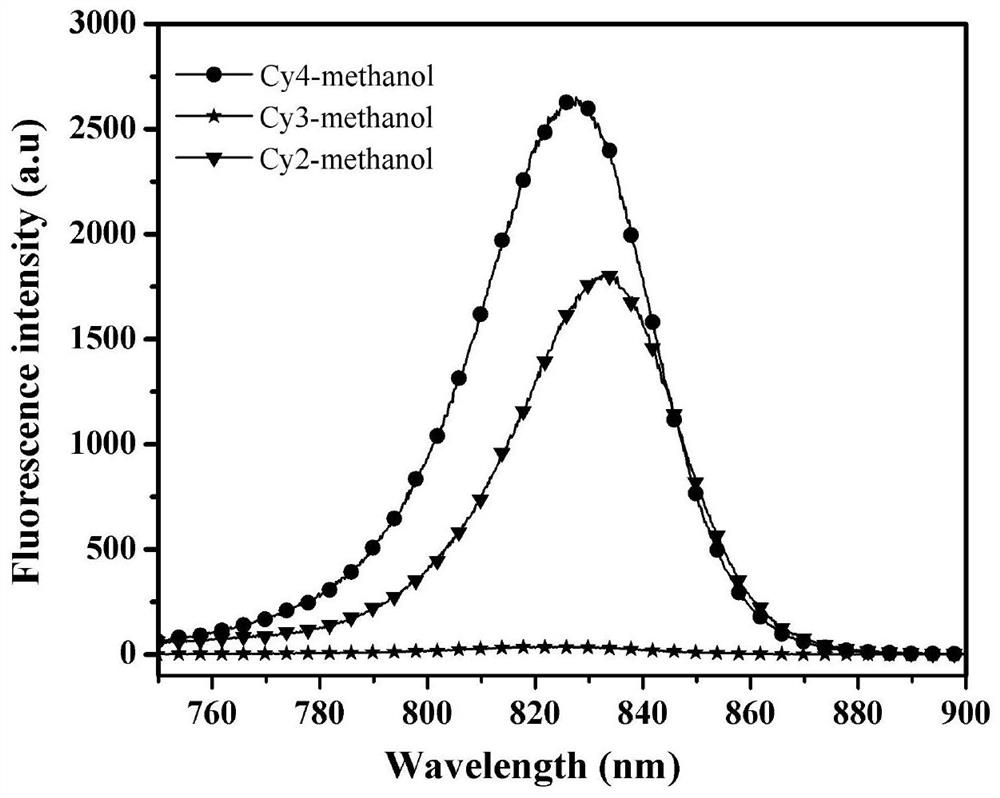
Heptamethine indocyanine dye as well as preparation method and application thereof
A technology of heptamethine indocyanine and indocyanine, which is applied in the field of heptamethine indocyanine dye and its preparation, can solve the problems of limited wide application, poor photostability, low photothermal conversion efficiency, etc., and achieves many chemical sites. , The effect of improving photothermal stability and clarifying biodegradation pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The invention provides the preparation method of above-mentioned heptamethine indocyanine dye, comprises the following steps:
[0077] ①When R 1 for When, the preparation method of described heptamethine indocyanine dye comprises the following steps:
[0078] A compound having a structure shown in formula a is reacted with a compound having a structure shown in formula b to obtain a compound having a structure shown in formula c;
[0079]
[0080] A compound having a structure shown in formula c undergoes a nucleophilic substitution reaction with a nucleophile to obtain a heptamethine indocyanine dye;
[0081] The nucleophile has a structure shown in formula d-1, formula d-2 or formula d-3;
[0082]
[0083] In the present invention, the preparation method of the compound having the structure shown in formula a preferably includes the following steps:
[0084] Phosphorus oxychloride is mixed with N'N-dimethylformamide to obtain Vilsmeier-Haack weak nucleophil...
Embodiment 1
[0133] (1) Preparation of compound (compound 1) shown in formula a
[0134] Add 1 mL of POCl to 1.2 mL of anhydrous DMF at 2 °C 3 After reacting for 30min, add 0.5mL cyclohexanone, heat and reflux for 1h; then cool to 20°C, add 2.9mL aniline / ethanol mixture dropwise, the molar ratio of aniline and ethanol in the mixture is 1:1; after stirring for 1.5 hours, pour into concentrated HCl, recrystallized in an ice-water bath, filtered, washed, and vacuum-dried to obtain 1.044 g of compound 1 with a yield of 61%.
[0135] LCMS(ESI+): Calculated [M+H] + 323.1, measured to 323.1.
[0136] Synthetic route is as shown in formula D:
[0137]
[0138] (2) Preparation of compound (compound 2) shown in formula b
[0139] Toluene, 1.3g 1,1,2-trimethyl-1H-benzo[e]indole and 1.1mL 1,3-propane sultone were heated to reflux for 18 hours; cooled to room temperature, the obtained blue crystals were filtered , washed with diethyl ether, the product was recrystallized with methanol and dieth...
Embodiment 2
[0154] Weigh 200 mg heptamethine indole cyanine dye Cy1, add 10 mL of a mixture of trifluoroacetic acid (TFA) and water, stir at room temperature for 4 hours, evaporate the solvent to dryness, add ethyl acetate and stir, filter and dry under vacuum The crude product was obtained, and then separated and purified by column chromatography to obtain the heptamethine indocyanine dye Cy2.
[0155] LCMS(ESI+): Calculated [M+H] + 886.3; 886.3 detected.
[0156] The synthetic route is shown in formula H:
[0157]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com