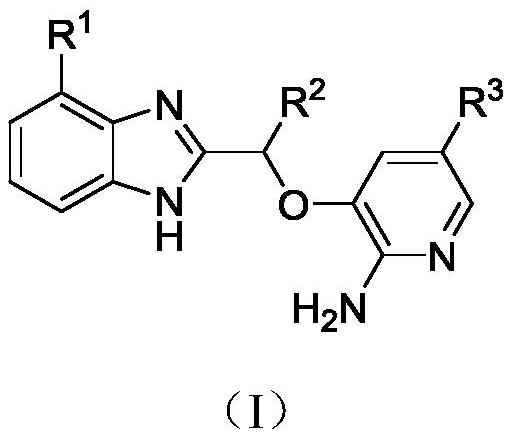
Compounds containing benzimidazole structure as well as preparation method and application thereof
A compound and benzene ring technology, applied in the preparation of ROS1 inhibitors, compounds containing benzimidazole structures and their preparation fields, can solve the problems of not having high selectivity, easily lead to drug resistance, etc., and achieve improved treatment safety, The effect of reducing off-target effects and reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
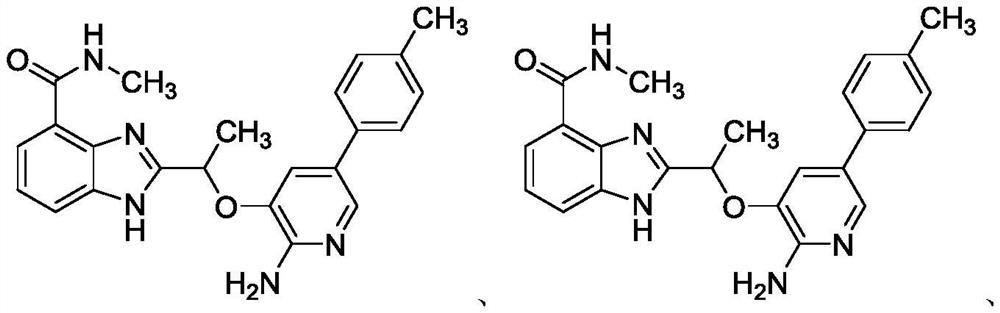
[0038] 2-(1-((2-amino-5-(4-methylphenyl)pyridin-3-yl)oxy)ethyl)-N-methyl-1H-benzo[d]imidazole-4- Formamide (I-1:R 1 =CONHCH 3 , R 2 =CH3 , R 3 =4-methylphenyl) synthesis
[0039] Synthesis of ethyl 2-((5-bromo-2-nitropyridin-3-yl)oxy)propionate (IV-1)
[0040] Dissolve compound II (5g, 22.8mmol) in 50mL N,N-dimethylformamide, cool in an ice bath, add sodium hydride (1.10g, 0.0274mol), stir, remove the ice bath, slowly add 2-bromo Ethyl acetate (III-1) (1.50mL×3, 34.20mmol), after the dropwise addition, was heated to 80°C for a constant temperature reaction for 12h, TLC (petroleum ether:ethyl acetate=4:1) detected that the reaction of the raw materials was complete. Stop heating, after cooling to room temperature, add 120mL of water and stir, extract with ethyl acetate (30mL×3), combine organic layers, wash with saturated sodium chloride solution (30mL×2), dry over anhydrous magnesium sulfate, suction filter, reduce The solvent was removed by pressure evaporation, and pur...
Embodiment 2
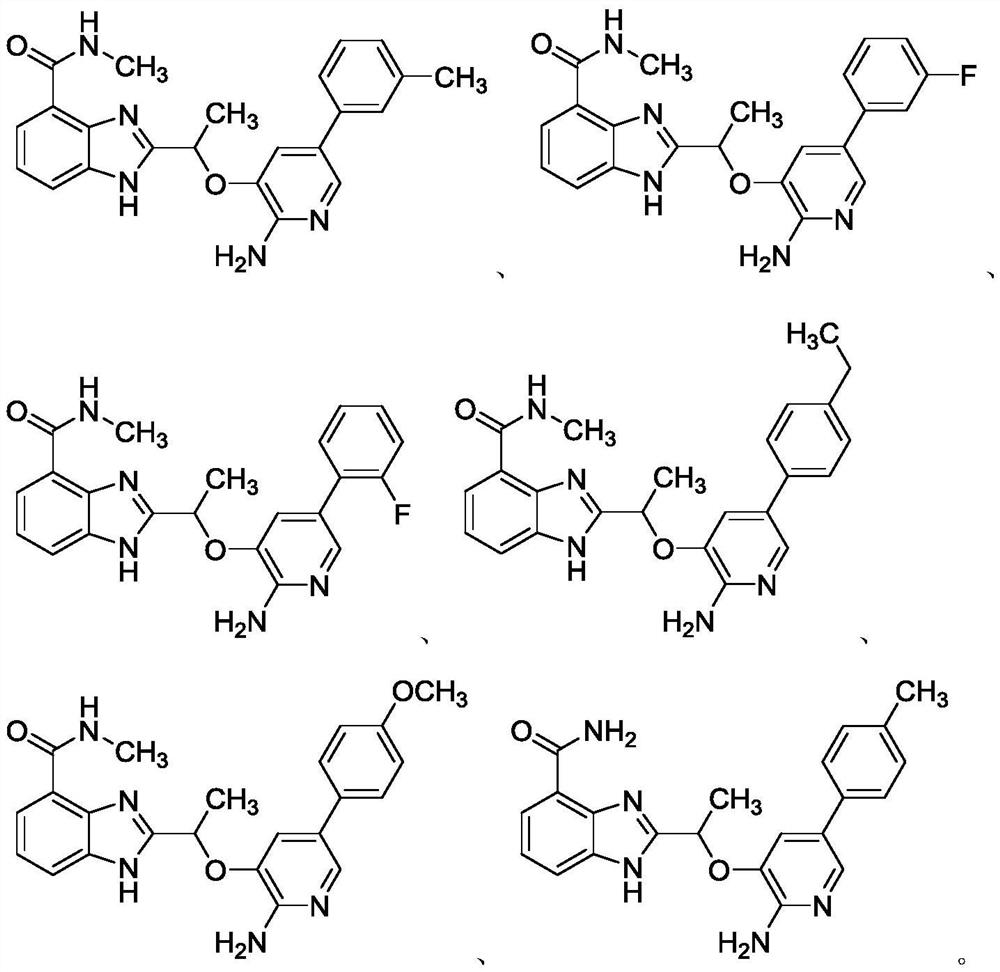
[0050] 2-(1-((2-amino-5-(4-fluorophenyl)pyridin-3-yl)oxy)ethyl)-N-methyl-1H-benzo[d]imidazole-4-carba Amide (I-2:R 1 =CONHCH 3 , R 2 =CH 3 , R 3 =4-fluorophenyl) synthesis
[0051] Synthesis of ethyl 2-((5-(4-fluorophenyl)-2-nitropyridin-3-yl)oxy)propionate (VI-2)
[0052] Dissolve IV-1 (0.50g, 1.57mmol) and 4-fluorophenylboronic acid (V-2) (0.33g, 2.35mmol) in 10mL of tetrahydrofuran, and dissolve potassium carbonate (0.61g, 4.39mmol) in 1mL of water to configure Potassium carbonate solution was added dropwise to the reaction solution, then tetrakis(triphenylphosphine)palladium (0.18g, 0.16mmol) was added, the reaction was refluxed under nitrogen protection for 24h, and the raw material was detected by TLC (petroleum ether:ethyl acetate=4:1) The response is complete. Heating was stopped, cooled to room temperature, the reaction system was evaporated to remove the solvent under reduced pressure, 10 mL of ethyl acetate and 15 mL were added for layering, the aqueous phase...
Embodiment 3
[0060] 2-(1-((2-amino-5-(3-methylphenyl)pyridin-3-yl)oxy)ethyl)-N-methyl-1H-benzo[d]imidazole-4- Formamide (I-3:R 1 =CONHCH 3 , R 2 =CH 3 , R 3 =3-Methylphenyl) Synthesis
[0061] Synthesis of Ethyl 2-((2-nitro-5-(3-methylphenyl)pyridin-3yl)-oxy)propionate (VI-3)
[0062] Dissolve IV-1 (1.00g, 3.13mmol) and 3-methylphenylboronic acid (V-3) (0.64g, 4.70mmol) in 20mL of tetrahydrofuran, and dissolve potassium carbonate (1.22g, 8.77mmol) in 2mL of water to configure Potassium carbonate solution was added dropwise to the reaction solution, then tetrakis(triphenylphosphine)palladium (0.36g, 0.33mmol) was added, the reaction was refluxed under nitrogen protection for 24h, and the raw material was detected by TLC (petroleum ether:ethyl acetate=4:1) The response is complete. Heating was stopped, cooled to room temperature, the reaction system was evaporated to remove the solvent under reduced pressure, 15 mL of ethyl acetate and 20 mL were added for layering, the aqueous phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com