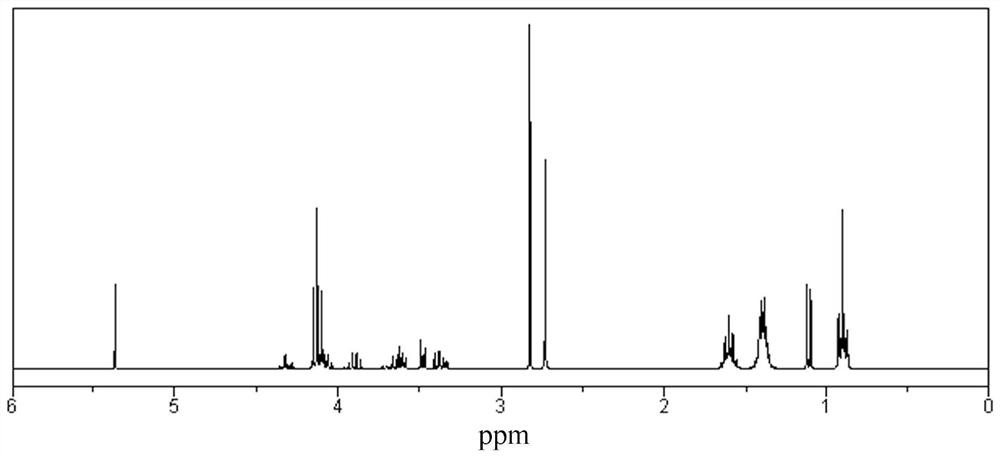
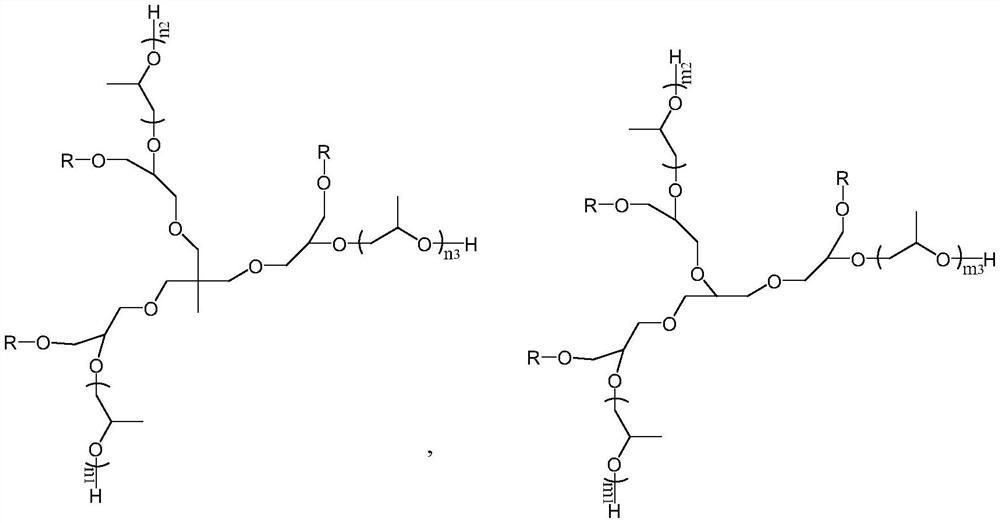
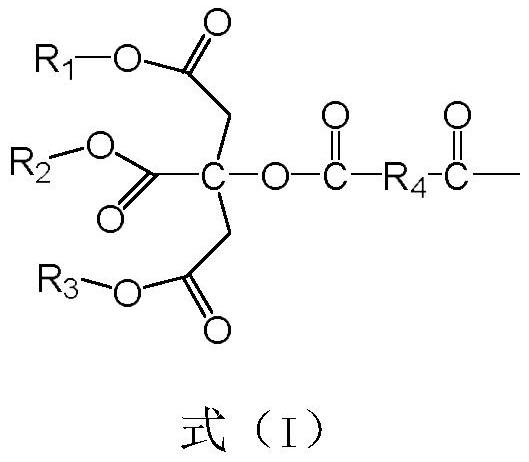
Citric acid triester modified polyether polyol and application thereof in polyurethane waterproof coating
A technology of polyether polyol and citric acid three, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of easy exudation of citrate esters and decreased performance stability of polyurethane materials, and achieves good encapsulation effect and good performance. The effect of leveling and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of tributyl citrate: add 30mol citric acid, 115mol butanol, 110mol water-carrying agent cyclohexane and Quality is the 2.5% p-benzenemethanesulfonic acid of the total mass of citric acid and alcohol and is stirred and heated to 150 ℃, reflux reaction 4h, is cooled to room temperature, product removes excess alcohol and water-carrying agent cyclohexane through decompression distillation, then The catalyst was removed by washing with 3% sodium bicarbonate solution, and finally dried to obtain tributyl citrate.
[0053] (2) Preparation of succinic anhydride acylated tributyl citrate: add 80mol carbon tetrachloride, 50mol tributyl citrate and 50mol butanediol acid anhydride, stirred and heated up to 80°C, reacted for 5h, cooled to room temperature, and the product was distilled to remove the solvent to obtain succinic anhydride acylated tributyl citrate.
[0054] (3) The preparation of tributyl citrate modified trihydric alcohol: add 90mol succinic anhydrid...
Embodiment 2
[0058] (1) Preparation of tripentyl citrate: add 30mol citric acid, 120mol amyl alcohol, 115mol water-carrying agent cyclohexane and 1.0% of the total mass of citric acid and alcohol is heated to 120° C. with stirring for 1.0% p-benzenemethanesulfonic acid, refluxed for 8 hours, and cooled to room temperature. The product is distilled under reduced pressure to remove excess alcohol and water-carrying agent cyclohexane, and then The catalyst was removed by washing with 1% sodium bicarbonate solution, and finally, tripentyl citrate was obtained by drying.
[0059] (2) Preparation of glutaric anhydride acylated tripentyl citrate: add 80mol carbon tetrachloride, 50mol tripentyl citrate and 50mol pentamyl citrate in a reaction kettle equipped with a temperature-controlled oil bath, condenser tube and stirrer acid anhydride, stirred and heated to 50°C, reacted for 8 hours, cooled to room temperature, and the product was distilled to remove the solvent to obtain glutaric anhydride ac...
Embodiment 3
[0064] (1) Preparation of trioctyl citrate: add 30mol citric acid, 110mol octanol, 115mol water-carrying agent cyclohexane and The quality is 4.0% p-benzenemethanesulfonic acid of the total mass of citric acid and alcohol is heated to 130 ℃ with stirring, reflux reaction 5h, is cooled to room temperature, product is removed excess alcohol and water-carrying agent cyclohexane through decompression distillation, then The catalyst was removed by washing with 2% sodium bicarbonate solution, and finally dried to obtain trioctyl citrate.
[0065] (2) Preparation of suberic anhydride acylated trioctyl citrate: add 100mol of carbon tetrachloride, 60mol of trioctyl citrate and 60mol of octane di acid anhydride, stirred and heated to 75°C, reacted for 6 hours, and the product was distilled to remove the solvent to obtain suberic anhydride acylated trioctyl citrate.
[0066] (3) Preparation of trioctyl citrate modified trihydric alcohol: add 90mol suberic anhydride acylated trioctyl cit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com