A kind of preparation method of ginsenoside f1
A technology of ginsenoside and P12H, which is applied in the fields of botanical equipment and methods, biochemical equipment and methods, enzymes, etc., can solve the problem of no synthesis, no synthesis yet, and no complete dammarane-type tetracyclic triterpenoid saponins found. access and other issues, to achieve the effect of easy planting, efficient and convenient access, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
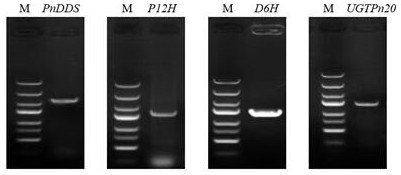
[0031] Embodiment 1: PnDDS , P12H , D6H and UGTPn20 gene cloning
[0032] Using Panax notoginseng root as material, grind Panax notoginseng root into powder with liquid nitrogen, then transfer it into a centrifuge tube, extract total RNA from Panax notoginseng root by guanidine isothiocyanate method, reverse transcription to synthesize the first strand of cDNA, the reaction system And the operation process is as follows: take 5μg TotalRNA, add 50ng oligo (dT), 2μL dNTP (2.5mM each), DEPC water in turn to the reaction volume of 14.5μL; after mixing, heat denaturation at 70°C for 5min, then quickly cool on ice 5 min, then add 4 μL 5×First-standbuffer, 0.5 μL LRNasin (200 U), 1 μL M-MLV (200 U) in sequence, mix well, centrifuge for a short time, incubate at 42 °C for 1.5 h, take out and heat at 70 °C for 10 min to terminate the reaction; The synthesized first-strand cDNA was used as a template, and ginsenoside F was amplified by PCR 1 Four genes on the synthetic pathway ...
Embodiment 2
[0043] Example 2: Plant expression vector construction
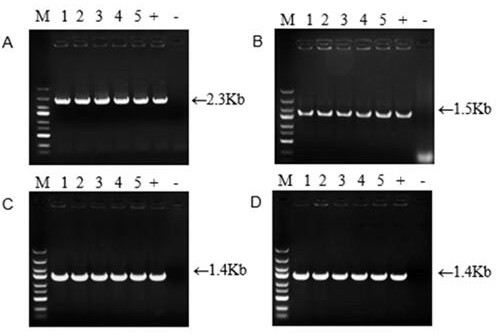
[0044] The plant expression vector pCAMBIA2300s was linearized with restriction endonucleases. The restriction enzyme digestion system was 15 μL pCAMBIA2300s plasmid, 5 μL 10×M buffer, 2.5 μL and 25 μL ddH for each of the enzymes at the front and rear restriction sites. 2 O, after mixing, centrifuge for a short time, and place it at 37°C for digestion for 3.5 hours; spot the digestion product on agarose gel for electrophoresis, and then perform gel recovery on the large fragment of the pCAMBIA2300s vector. The whole process uses SanPrep column DNA gel Recovery kit (Shanghai Shenggong); take 1 μL of the recovered product to detect the size and concentration of the recovered fragments by agarose gel electrophoresis, and store at -20°C for later use. During homologous recombination, the ClonExpressMultiS One Step Cloning Kit was used for assembly, and the recovered PnDDS , P12H , D6H and UGTPn20 Connected to pCAMBIA2...
Embodiment 3
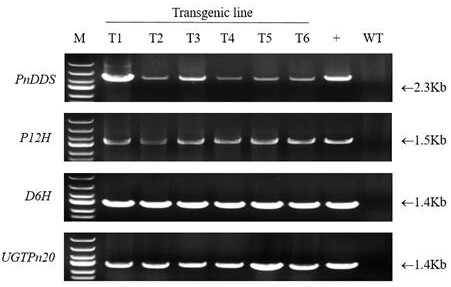
[0046] Example 3: Agrobacterium-mediated plant genetic transformation and screening of transgenic plants
[0047] The above-constructed plant expression vector pCAMBIA2300s- DS , pCAMBIA2300s- P12H , pCAMBIA2300s- D6H and pCAMBIA2300s- UGTPn20 Transfer to Agrobacterium tumefaciens LBA4404 competent cells. The operation steps are: take 2 μg of plasmid into a centrifuge tube containing 200 μL of competent cells, mix gently, ice bath for 5 minutes, and then transfer to liquid nitrogen for freezing for 1 minute, and then quickly Place in a water bath at 37°C for 5 minutes, then immediately in an ice bath for 2 minutes, add 800 μL of LB liquid medium, and incubate at 28°C and 200 rpm for 4 hours with shaking; apply the activated Agrobacterium to a solution containing 50 mg / L kanamycin and 25 mg / L lysine. On the LB solid medium of Fuping, stand at 28 °C for about 48 hours; select a single colony and shake the bacteria, and shake them in LB liquid medium containing 50 mg / L kan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com