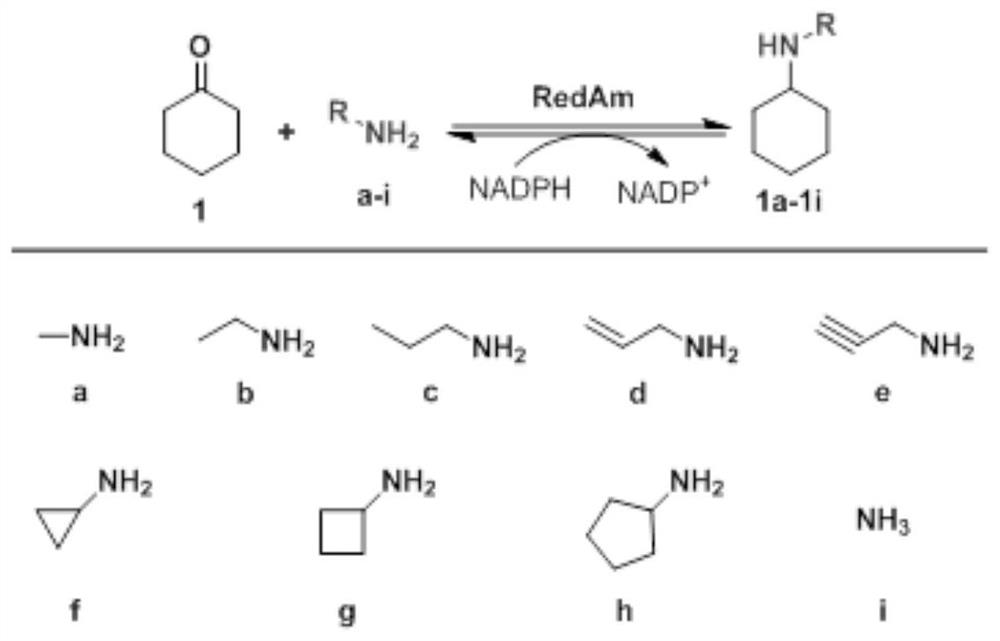
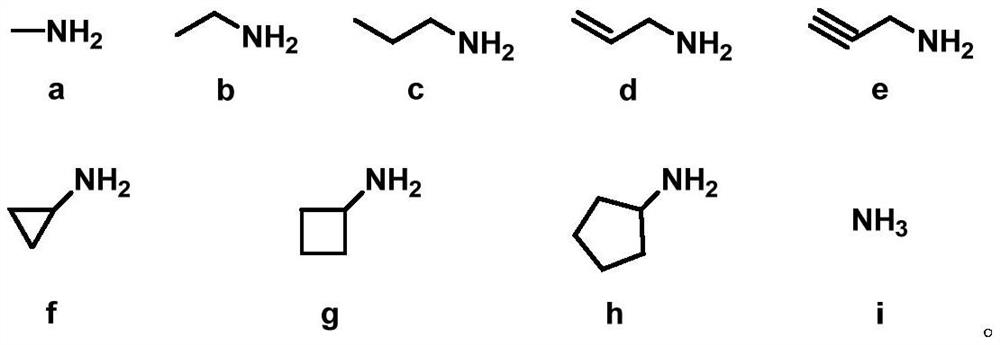
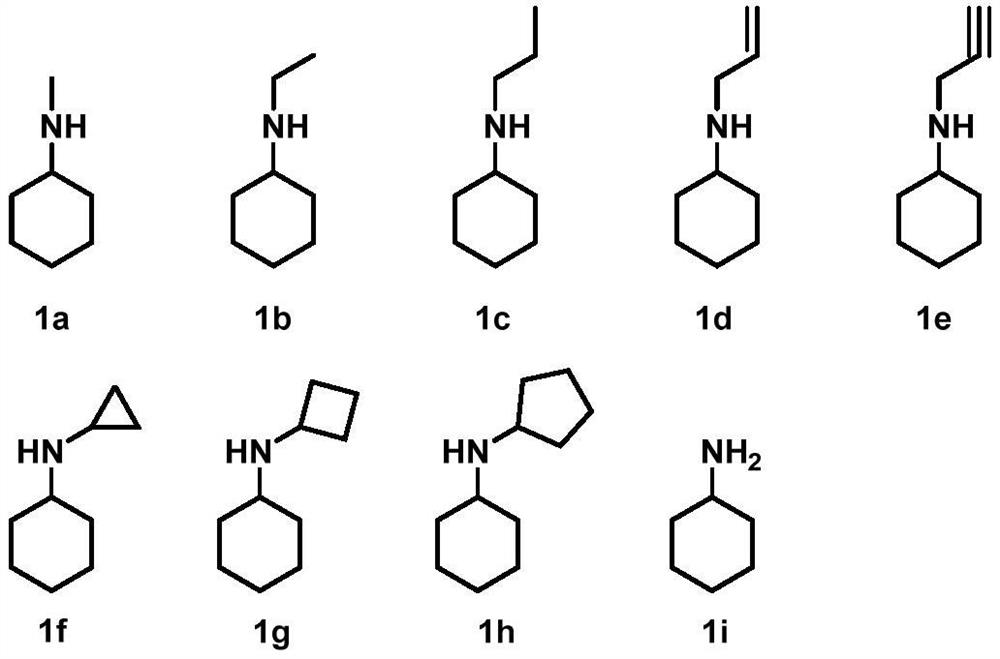
Reductive aminase and application thereof in secondary amine synthesis
A reductive amination enzyme and reductive amination enzyme catalyst technology, applied in the field of bioengineering, can solve the problems of poor operational stability and inactivation of reductive amination enzymes, and achieve increased industrial application value, high-efficiency synthesis, and reductive amination enzyme activity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The cultivation of the Rhizobium genus whose strain preservation number of embodiment 1 is ACCC 19914
[0058] Rhizobia with strain preservation number ACCC 19914 was inoculated into 50 ml of LB medium (peptone 10 g / L, yeast extract 5 g / L, sodium chloride 10 g / L), and cultured at 37° C. for 2-4 days. The culture solution was centrifuged at 8000rpm to collect the bacterial cells. The collected bacterial pellets were used immediately, or stored in a 4°C refrigerator for use.
Embodiment 2
[0059] Example 2 Gene Cloning of Reductive Aminase RsRedAm
[0060] The invention adopts the genome extraction method of phenol-chloroform method to extract the complete genome of the bacteria. The bacterial cell precipitates collected in Example 1 were placed in a mortar respectively, and an appropriate amount of liquid nitrogen was added for quick-freezing, rapid grinding, and the freeze-dried bacterial cells were ground to whitish powder. Transfer the powdered bacteria to a 2mL centrifuge tube, add 700μL TE buffer to resuspend the bacteria, add 700μL phenol: chloroform: isoamyl alcohol (25:24:1), gently invert and mix for 10min, centrifuge at 10000rpm for 10min, draw Transfer the supernatant to another clean 1.5ml centrifuge tube. Add 700 μL of phenol:chloroform:isoamyl alcohol (25:24:1) again, mix gently by inversion for 10 minutes, centrifuge at 10,000 rpm for 10 minutes, and pipette the supernatant into another clean 1.5ml Eppendorf tube with the tip of a pipette cut of...
Embodiment 3
[0065] Example 3 Preparation of Reductive Aminase RsRedAm Recombinant Expression Transformant
[0066] The target fragment of the reductive aminase RsRedAm amplified by PCR in Example 2 and the pET 28a empty plasmid were double-digested overnight with restriction endonucleases Nde I and Xho I at the same time; then purified by agarose gel electrophoresis, DNA reagent box recycling. The recovered target fragment and the empty vector were ligated under the action of T4 DNA ligase at 16° C. for 12 hours to obtain the recombinant plasmid pET28a-(RsRedAm).
[0067] Transform the obtained recombinant plasmid into E.coli BL21(DH3), spread it on the LB medium plate containing 50 μg / ml kanamycin, and cultivate it at 37°C for 8 hours, carry out colony PCR verification on the grown colonies, pick Colony PCR amplified positive clones with a target band of about 1000 bp in length. After sequencing and verification, the corresponding plasmids were extracted, further transformed into E.col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com