Hybridoma cell strain secreting ketoconazole monoclonal antibody and application of hybridoma cell strain
A hybridoma cell line and ketoconazole single technology, applied in the field of immunochemistry, can solve the problem that the sensitivity of ketoconazole antibody needs to be further improved, and achieve the effects of good detection sensitivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of ketoconazole hapten
[0043] Since the small molecule of ketoconazole is not immunogenic and cannot stimulate the immune response of mice to produce antibodies, it is necessary to couple ketoconazole to the protein through protein linkage technology to obtain immunogenicity; protein coupling Active groups commonly used in the technology include amino, carboxyl, hydroxyl, mercapto, etc. Since the molecular structure of ketoconazole does not contain amino, carboxyl, or hydroxyl groups, carboxyl groups need to be derived from its structure.
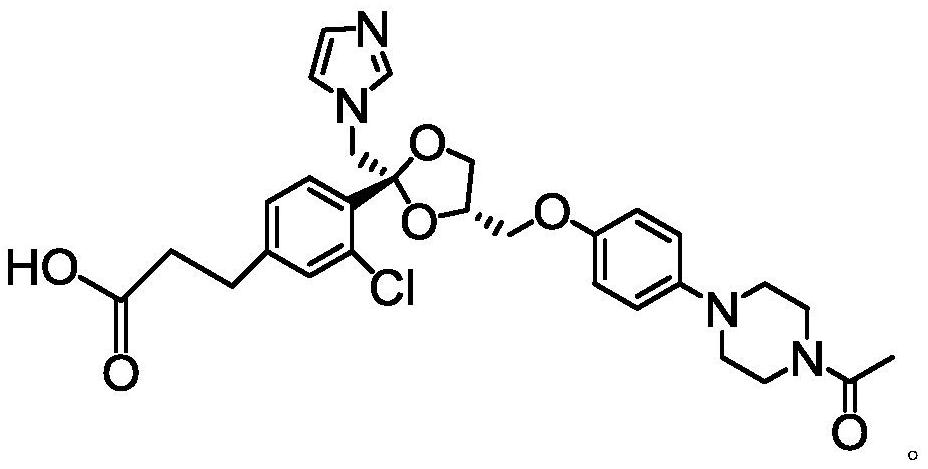
[0044] The ketoconazole hapten structure after derivation of the present invention is as follows:
[0045]
[0046] The derivatization process includes the following three reactions:
[0047]
[0048] 1. Weigh raw materials ketoconazole 1, Pd 2 (dba) 3 , cesium carbonate in a Schlenk tube, add PPh3 into the Schlenk tube, then add methyl acrylate 2, solvent 1,4-dioxane. Complete deoxygenation wa...
Embodiment 2
[0051] Embodiment 2: the synthesis of complete antigen of ketoconazole
[0052] Weigh 8 mg ketoconazole hapten (KCZ-HS), 5 mg N-hydroxysuccinimide (NHS), dissolve in 300 μL N,N-dimethylformamide (DMF), stir at room temperature for 10 min; weigh again Take 7mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), fully dissolve it with 100μL DMF, add it to the KCZ-HS solution, and stir at room temperature for 4-6h (called liquid A). Take 5mg of BSA, dilute it to 2mg / mL with 0.01M carbonate buffer solution (CBS) (called solution B), then slowly add solution A to solution B drop by drop, react at room temperature overnight; then use 0.01M PBS solution Dialyze to remove the unreacted small molecule hapten to obtain the complete antigen KCZ-HS-BSA, which is identified by ultraviolet absorption scanning method.
Embodiment 3
[0053] Embodiment 3: the synthesis of ketoconazole coating former
[0054] Dissolve 4 mg of ketoconazole hapten (KCZ-HS) and 2.5 mg of N-hydroxysuccinimide (NHS) in 300 μL of anhydrous N,N-dimethylformamide (DMF), and stir at room temperature for 10 min to obtain ketone Conazole hapten (KCZ-HS) solution; 4.2 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was dissolved in 100 μL of anhydrous DMF and added to KCZ -HS solution, stirred at room temperature and reacted for 4-6 hours to obtain liquid A; 5 mg of chicken ovalbumin (OVA) was diluted with 1 mL of carbonate buffer solution (CBS) with a concentration of 0.01 mmol / L to obtain liquid B; Slowly add solution A to solution B to react dropwise to obtain a reaction solution; dialyze the reaction solution with PBS solution to remove unreacted small molecule hapten to obtain the coating agent (KCZ-HS-OVA).
PUM
| Property | Measurement | Unit |
|---|---|---|
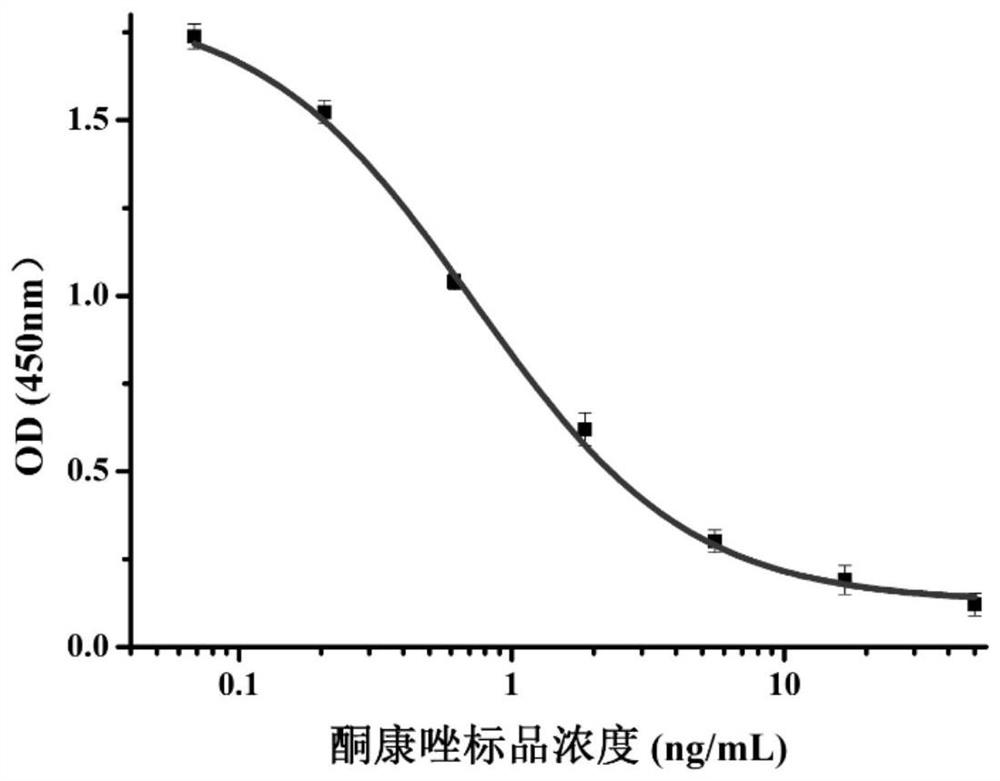
| Ic50 value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com