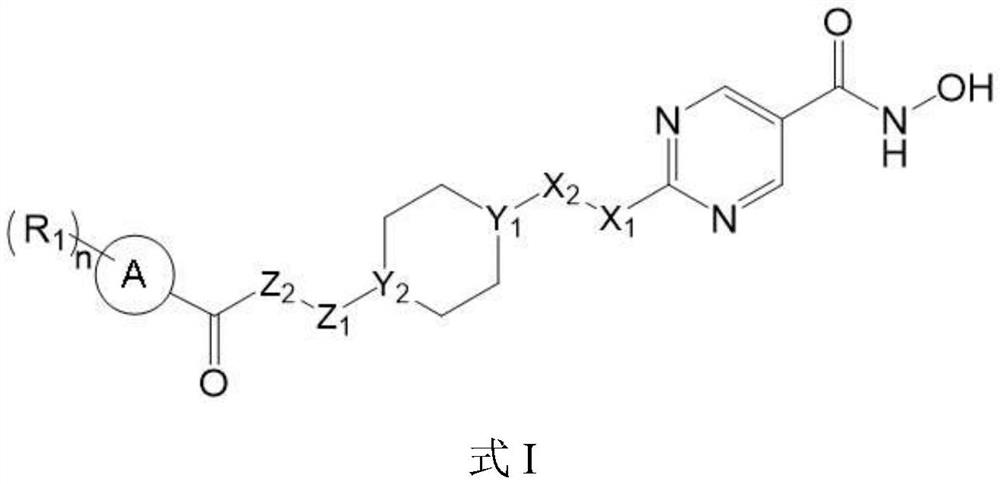
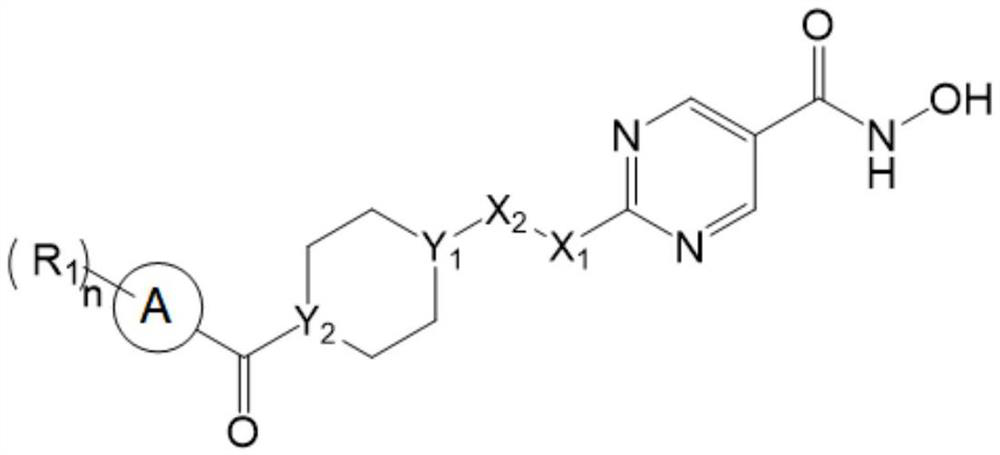
Hydroximic acid derivative and application thereof
A technology of compounds and hydrates, applied in the field of biomedicine, can solve problems such as drug interactions, toxic side effects, complex pharmacokinetics, etc., and achieve the effects of improving therapeutic effects, reducing adverse reactions, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0316] Example 1, 2-(4-((2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzamide)methyl)piperidine- 1-yl)-N-hydroxypyrimidine-5-carboxamide (compound 1)
[0317]
[0318] A: 4-Aminomethylpiperidin-1-yl-pyrimidine-5-carboxylic acid methyl ester
[0319] In a 500ml three-necked flask, add 200 milliliters of N,N-dimethylacetamide, 34.3 grams (0.3mol) of compound 4-aminomethylpiperidine and 65.5 milliliters (0.375mol) of N,N-diisopropylethyl Amine, at room temperature, add 25.9 g (0.15 mol) of methyl 4-chloropyrimidine-5-carboxylate dropwise while stirring in 100 ml of N,N-dimethylacetamide solution, after 3 hours, the reaction solution was slowly Pour into the stirred ice-water mixture, then extract three times with 300 ml ethyl acetate respectively, combine the organic phases, wash twice with an appropriate amount of saturated saline, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain light yellow Waxy. Then recrystallized wi...
Embodiment 2
[0332] Example 2, 2-(4-((2H-indazole-7-carboxamide)methyl)piperidin-1-yl)-N-hydroxypyrimidine-5-carboxamide (compound 2)
[0333]
[0334] A: Methyl 2-(4-((2H-indazole-7-carboxamide)methyl)piperidin-1-yl)-5-carboxylate
[0335] Into a 250ml one-necked flask, add 8.1 grams (50mmol) of the compound 2H-indazole-7-carboxylic acid, 100ml of N,N-dimethylformamide, stir at room temperature, and then add 8.1 grams of N,N-carbonyldiimidazole (50mmol), there was gas evolution, and after 2 hours, 12.5 g (50mmol) of 4-aminomethylpiperidin-1-yl-pyrimidine-5-carboxylic acid methyl ester was added. Stir overnight, and the next day, slowly pour the reaction solution into the stirred ice-water mixture, and a light yellow solid precipitates out. After standing for 3 hours, filter, wash with water, and dry to obtain a light yellow powder product, which is the target compound 17.2 g was directly used in the next reaction without further purification. ESI-MS: m / z 395.2.
[0336] B: 2-(4-((2H-i...
Embodiment 3
[0346] Example 3, 2-((1-((2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperidine-4- base) methylamino)-N-hydroxypyrimidine-5-carboxamide (compound 3)
[0347]
[0348] A: Methyl 2-(piperidin-4-ylmethylamino)pyrimidine-5-carboxylate
[0349] In a 100ml single-necked bottle, add 4.0g of 2-((1-(tert-butoxycarbonyl)piperidin-4-yl)methylaminopyrimidine-5-carboxylic acid methyl ester, and 40ml of ethyl acetate hydrochloric acid gas solution, room temperature Under stirring reaction, TLC monitors the reaction process, after the reaction is complete, the precipitated solid is filtered to obtain 2.8 g of the target product.
[0350] B: 2-((1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperidin-4-yl)methanol Amino)pyrimidine-5-carboxylic acid methyl ester
[0351] In a three-necked flask, add 5.96 grams of 2-fluoro-5-(4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid, 100ml of N,N-dimethylformamide, At room temperature, under stirring, add 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com