Organic phosphorus ligand as well as preparation method and application thereof
A phosphine ligand, an independent technology, applied in the field of organophosphorus ligand and its preparation, can solve the problems of long process route, corrosive reaction system, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Aiming at the deficiencies of the prior art, the present invention provides a novel phosphine ligand and its preparation method. The mixture of synthesis gas and methanol can obtain fuel in one pot under the condition of mixing the phosphine ligand and the Rh / Ru bimetallic catalyst precursor. Ethanol, through process optimization, the reaction can be carried out under mild conditions, and the selectivity is high, which can greatly reduce the cost, and has a good industrialization prospect, huge economic benefits and social value.
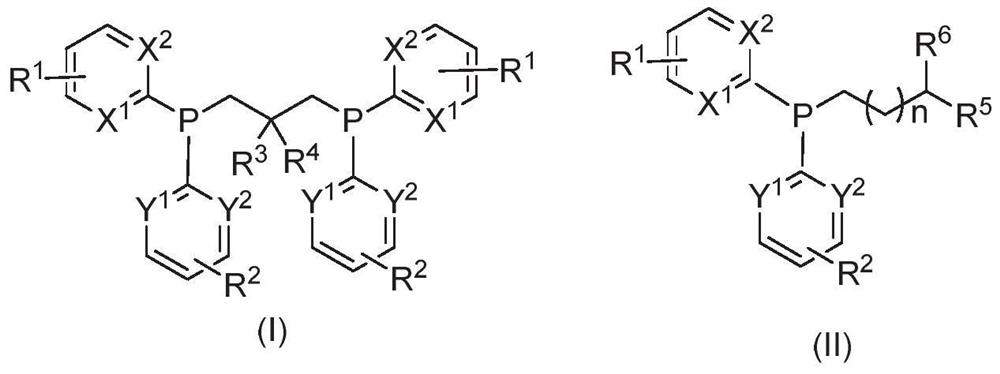
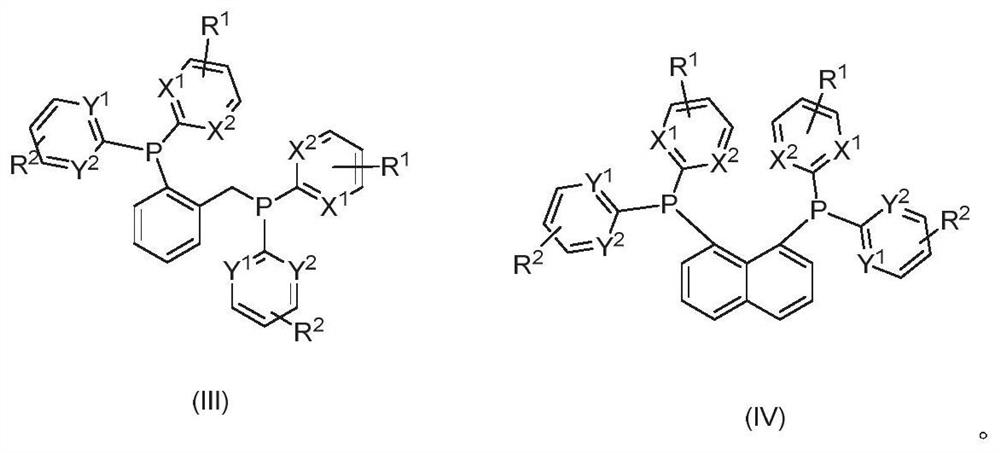
[0025] In view of the deficiencies in the prior art, the object of the present invention is to provide a novel phosphine ligand, which has a chemical structure as shown in the following formula (I) or formula (II),
[0026]
[0027] in,
[0028] x 1 、X 2 , Y 1 , Y 2 each independently N or CR 6 ;
[0029] R 1 , R 2 , R 6 independently H, F, Cl, Br, I, cyano, trifluoromethyl, alkyl, alkenyl, alkoxy, alkylmercapto, dialkylamino, ami...
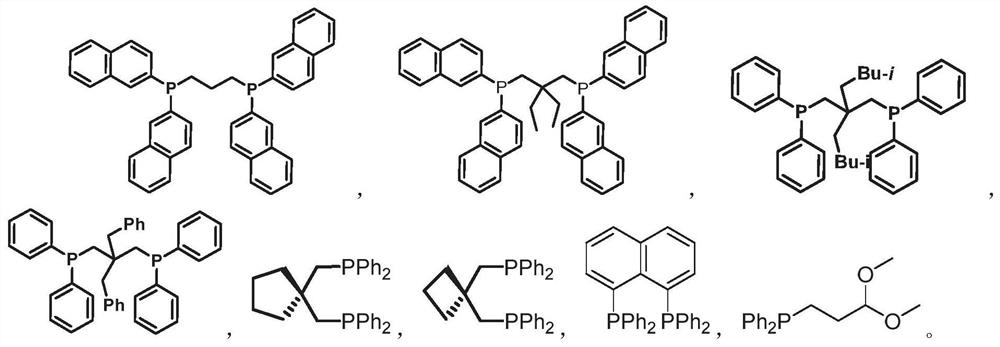
Embodiment 1
[0088] The general preparation method is as follows
[0089]
[0090] At room temperature, add 10 to 12.5 mmol of bisarylphosphine hydrogen 2 into a 250 ml three-necked flask, vacuumize and fill with argon, repeat the process 3 times, add 30 mL of dry THF (tetrahydrofuran) as a solvent in an argon atmosphere, and then The three-necked flask was placed in an ice bath, and 15 mmol of n-butyllithium (1.6M n-hexane solution) was slowly added dropwise. The reaction solution changed from clear to dark red solution, indicating that P-Li had been generated, and the addition was completed. After that, make it return to room temperature naturally and continue to stir for 2 hours; then, under room temperature, add gem-dialkyl-1,3-diX propane 1 (5mmol) dropwise in THF solution, monitor until after the reaction ends, use Quench the reaction with degassed saturated ammonium chloride aqueous solution, extract with dichloromethane, dry over anhydrous sodium sulfate, remove volatile reagent...
Embodiment 2
[0096]
[0097] Under room temperature conditions, weigh 1.0 times the equivalent of 1,8-dibromonaphthalene in a 250 ml three-necked flask, vacuumize and fill with argon, repeat 3 times, add 150 mL of dry Et in an inert gas O (diethyl ether) as a solvent, and then The three-necked reaction flask was placed in an ice bath, and 2.2 times of equivalent n-butyl lithium (1.6M in n-hexane) was slowly added dropwise. After the dropwise addition, it was naturally returned to room temperature and continued to stir for 2 hours; subsequently, in Under ice-bath conditions, 2.2 times the equivalent of diphenylphosphine chloride solution was added dropwise. After the reaction was completed, the reaction was quenched with degassed saturated ammonium chloride aqueous solution, extracted with dichloromethane, dried over anhydrous sodium sulfate, and decompressed. The volatile reagents were removed under certain conditions, and finally, a short chromatographic separation was carried out quick...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com