Preparation method and application of titanate-based hollow material
A titanate, layered titanate technology, applied in the directions of alkaline earth metal titanate, titanate, chemical instruments and methods, etc., can solve the problems of harsh conditions, lack of material stress, poor cycle performance, etc. Achieve the effect of simple preparation process, lower production cost, and improved capacity characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
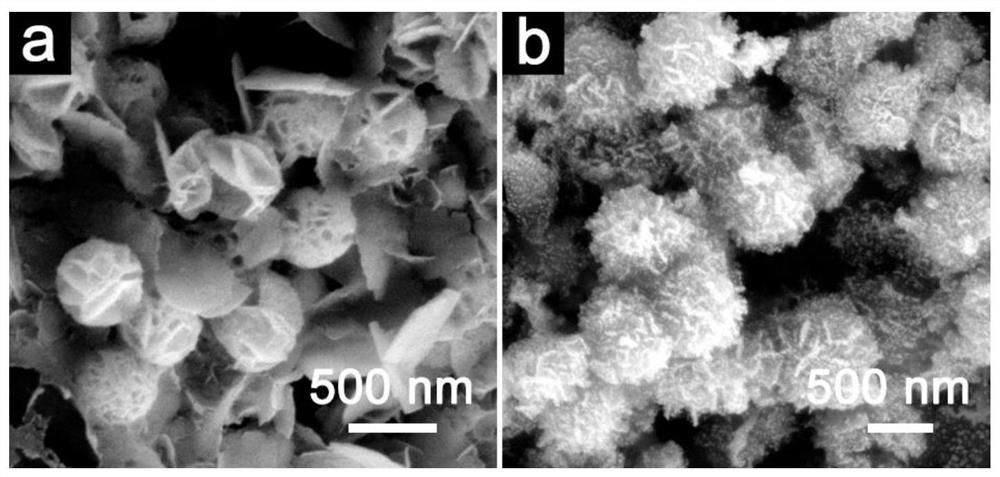
[0055] Lithium titanate is the first hydrothermal product to prepare magnesium titanate hollow nanospheres:
[0056] Add 18mL of ammonia water and 2.6mL of ethyl orthosilicate into 30mL of ethanol, and react at room temperature for 60min to obtain silica nanospheres. Add 0.6 g of the above-mentioned silica nanospheres to 40 mL of ethanol, add 0.6 g of hexadecylamine, 10 mL of ammonia water and 5 mL of isopropyl titanate and react for 20 min to obtain amorphous titanium dioxide-coated silica core-shell nanospheres. The diameter of the titanium dioxide-coated silicon dioxide core-shell nanosphere is about 500 nm, and the thickness of the titanium dioxide shell layer is about 50 nm.
[0057] The above-mentioned amorphous titania-coated silica core-shell nanospheres were placed in 0.4M LiOH solution for hydrothermal reaction, the reaction temperature was 160°C, and the reaction time was 6h to obtain a layered hollow lithium titanate nanosphere material . After washing and drying...
Embodiment 2
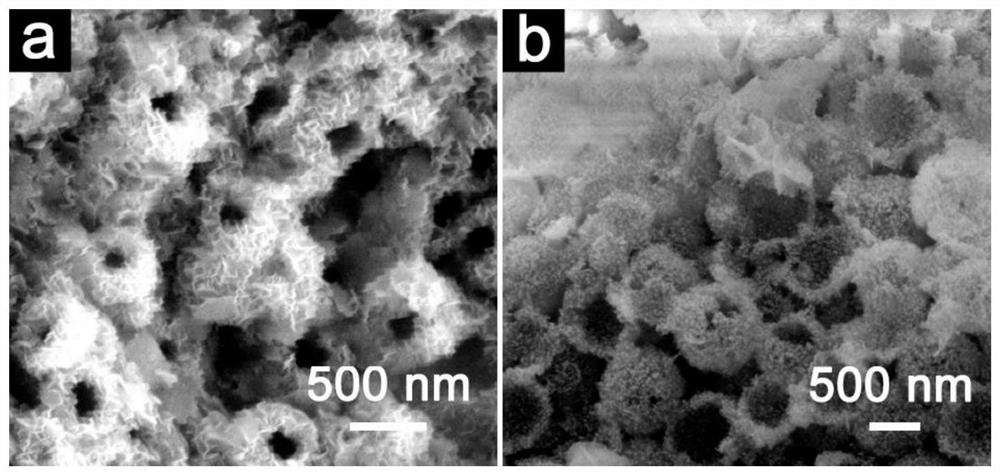
[0060] Lithium titanate is the first hydrothermal product to prepare calcium titanate hollow nanospheres:
[0061] Add 18mL of ammonia water and 2.6mL of ethyl orthosilicate into 30mL of ethanol, and react at room temperature for 60min to obtain silica nanospheres. Add 0.6 g of the above-mentioned silica nanospheres to 40 mL of ethanol, add 0.6 g of hexadecylamine, 10 mL of ammonia water and 5 mL of isopropyl titanate and react for 20 min to obtain amorphous titanium dioxide-coated silica core-shell nanospheres. The diameter of the titanium dioxide-coated silicon dioxide core-shell nanosphere is about 500 nm, and the thickness of the titanium dioxide shell layer is about 50 nm.
[0062] The above-mentioned amorphous titania-coated silica core-shell nanospheres were placed in 0.8M LiOH solution for hydrothermal reaction, the reaction temperature was 140°C, and the reaction time was 8h, and the layered hollow lithium titanate nanosphere material was obtained. . After washing a...
Embodiment 3
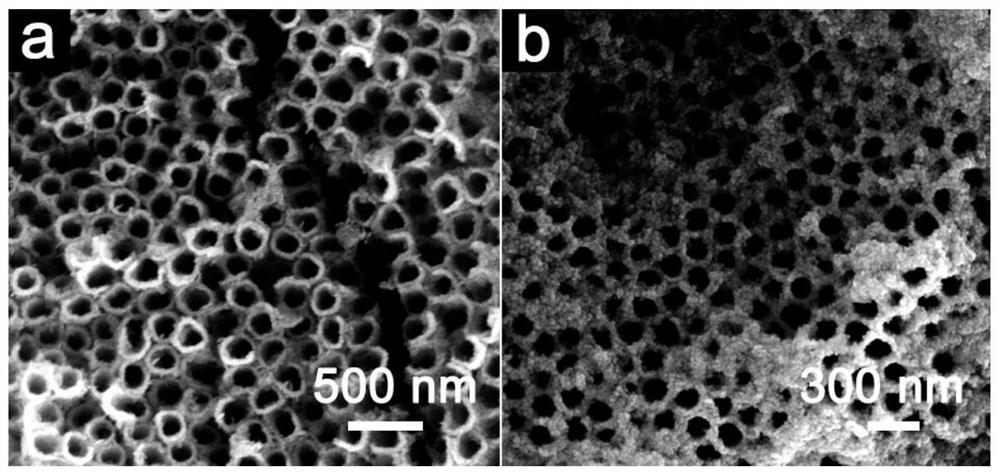
[0065] Lithium titanate is the first hydrothermal product to prepare magnesium titanate hollow nanotube arrays:
[0066] Titanium dioxide nanotube arrays were prepared by the anodic polarization method. The sodium fluoride solution with a concentration of 0.3M was used as the etchant, and the mixed solution of deionized water and ethylene glycol with a volume ratio of 1:8 was used as the solvent. The reaction time was 2 h, and the loading voltage was 50V, the titania nanotube array with amorphous structure was obtained, the inner diameter was 150nm, the outer diameter was 180nm, and the length was 4μm.
[0067] The TiO nanotube arrays with the above amorphous structure were placed in 0.3M LiOH·H 2 The O solution was subjected to hydrothermal reaction, the reaction temperature was 150° C., and the reaction time was 6 h, and the layered lithium titanate nanotube array material was obtained. After cleaning and drying, the layered lithium titanate nanotube arrays were placed in 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com