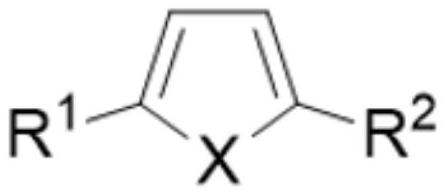
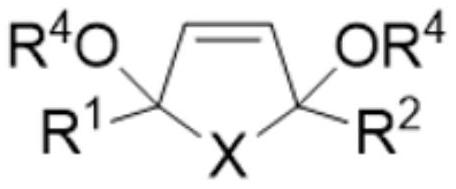
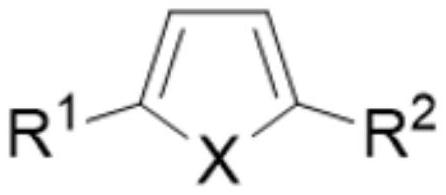
Method for electrochemically preparing five-membered heterocyclic dialkoxy compound
A five-membered heterocycle and dialkoxy technology, applied in the field of organic electrochemical synthesis, can solve the problems of high production cost, inconvenient large-scale production, and large discharge of three wastes, so as to reduce emissions and increase output per unit electrode area , the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Add 100g methyl alcohol, 10g thiophene, 0.05g tetrabutyl ammonium bromide and 10g dimethyl adipate in electrolytic cell, the base material of anode is titanium, and coating metal is selected from ruthenium and scandium, and wherein ruthenium content is 80wt%, The content of scandium is 20wt%, and the specific surface area of the electrode surface is 750m 2 / g, the cathode uses a lead electrode. Control the anode current density to 1000A / m 2 , the cathode current density is 1500A / m 2 , Electrolyzed at 2°C for 7h. The product yield is determined by gas chromatography. The yield of the anode product 2,5-dimethoxydihydrothiophene is 95%, and the current efficiency is 93%. The yield of the cathode product 1,6-hexanediol is 98%, and the current efficiency is 98%. 95%, the total current efficiency is 188%.
Embodiment 2
[0055] Add 100g methanol, 20g 2-n-propylthiophene, 0.2g tetraethylammonium iodide and 10g dimethyl succinate in the electrolytic cell, the base material of the anode is titanium, and the coating metal is selected from ruthenium and lanthanum, wherein ruthenium The content of lanthanum is 90wt%, the content of lanthanum is 10wt%, and the specific surface area of the electrode surface is 500m 2 / g, the cathode uses a cadmium electrode. Control the anode current density to 1500A / m 2 , the cathode current density is 1000A / m 2 , Electrolyzed at 0°C for 6h. The product yield is determined by gas chromatography. The yield of the anode product 2,5-dimethoxy-2-n-propyldihydrothiophene is 92%, the current efficiency is 94%, and the yield of the cathode product 1,4-butanediol The current efficiency is 95%, the current efficiency is 92%, and the total current efficiency is 186%.
Embodiment 3
[0057] Add 75g ethanol, 10g N-methyl-2,5-diethylpyrrole, 0.5g tetramethylammonium chloride and 20g diethyl suberate in the electrolytic cell, the base material of anode is titanium, and coating metal is selected From ruthenium and cerium, wherein the content of ruthenium is 98wt%, the content of cerium is 2wt%, and the specific surface area of the electrode surface is 1000m 2 / g, the cathode uses a titanium electrode. Control the anode current density to 500A / m 2 , the cathode current density is 2000A / m 2 , Electrolyzed at 10°C for 6h. The product yield is determined by gas chromatography. The yield of the anode product N-methyl-2,5-diethoxy-2,5-diethyldihydropyrrole is 91%, the current efficiency is 90%, and the cathode product is 1,8 - The yield of octane glycol is 92%, the current efficiency is 90%, and the total current efficiency is 180%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com