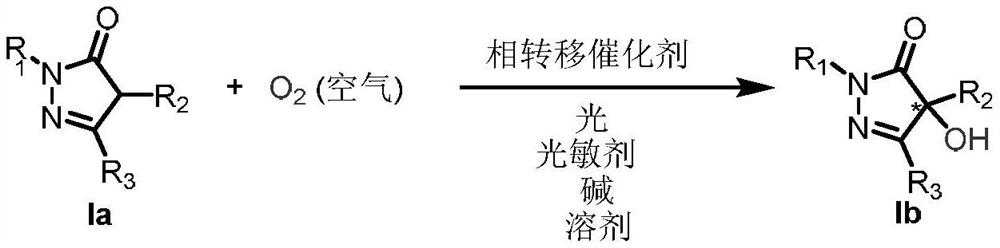
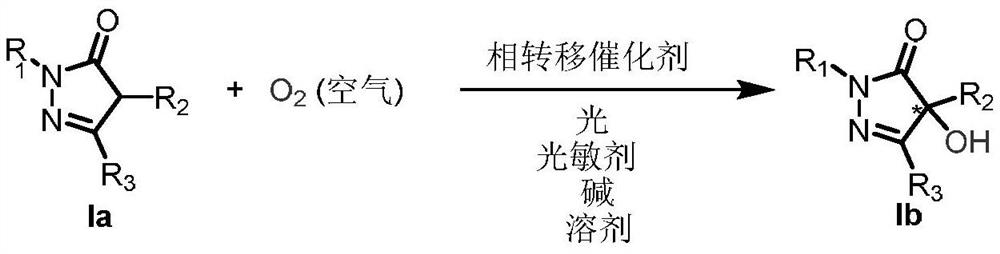
Method for realizing asymmetric alpha-hydroxylation of pyrazolone compounds through phase-transfer catalyzed photosensitized molecular oxygen
A technology of phase transfer catalysis and phase transfer catalyst, applied in organic chemistry and other directions, to achieve mild reaction conditions, good substrate applicability, environmental friendliness, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Preparation of Ia-1
[0020]
[0021] Add NaH (60% mass fraction, 8.4g) into a 250mL three-necked flask, connect the reflux device, and connect with argon protection. At room temperature, 17.6 ml of dimethyl carbonate and 30 ml of ultra-dry tetrahydrofuran were added to the system. Heat the system to reflux. Subsequently, 10ml of acetophenone was carefully added dropwise to the system, and bubbles were released. The dropwise addition was completed in half an hour, and the stirring reaction was continued for 2 hours until no acetophenone remained as detected by TLC. After the reaction, the temperature was lowered, and 20 ml of saturated ammonium chloride was carefully added to the system under an ice bath, and the pH was adjusted to 6-7 with 3 mol / L hydrochloric acid. Then add ethyl acetate to separate the layers, the aqueous layer was extracted once with ethyl acetate, the organic layers were combined, washed once with water, washed once with saturat...
Embodiment 37
[0030]
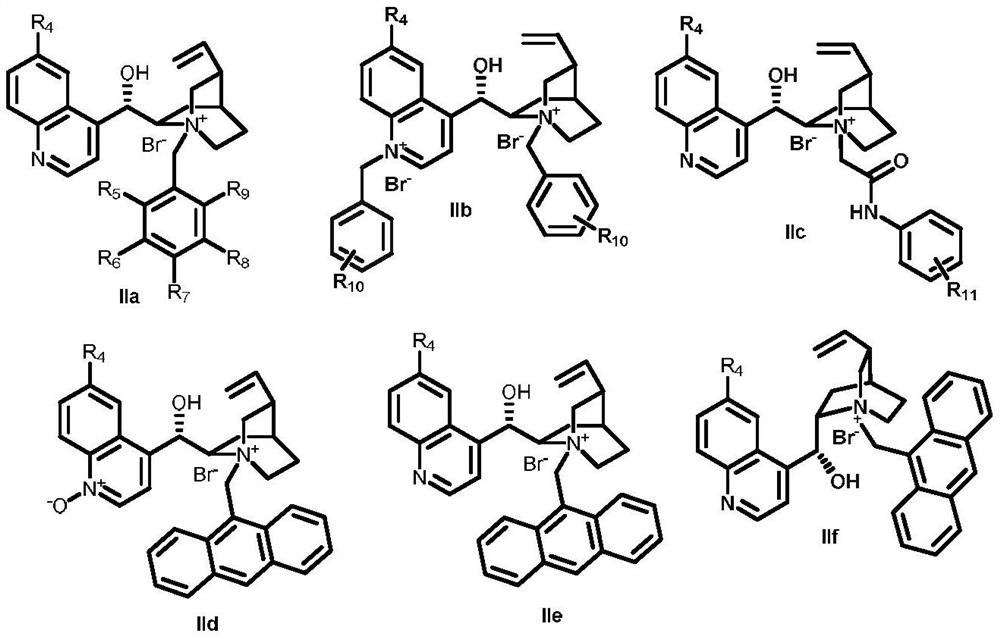
[0031] Take a 10mL single-port reaction flask, add 0.0324g (0.1mmol) pyrazolone substrate Ia-1, 0.0001g (tetraphenylporphyrin (TPP), and 0.0028g phase transfer catalyst IIa-1 respectively. At 15°C, Add 2 mL of toluene and stir for 10 minutes. Subsequently, add 0.2 mL of 30% K 2 CO 3 Aqueous solution, react under the irradiation of 3W LED white light. After 2 hours of reaction, TLC (thin layer chromatography) traced that the reaction was almost complete. The product was separated by petroleum ether / ethyl acetate=5:1 column chromatography with a yield of 89%. Chiral HPLC (high performance liquid chromatography) analysis, the final measured ee value was 15%. Chiral OJ-H column (250×4.6mm); mobile phase: n-hexane:isopropanol=9:1; flow rate 0.8mL / min; column temperature: 25°C; detection wavelength: 254nm. τ R (major)=12.6min,τ R (minor) = 10.7min. 1 H NMR (400MHz, Chloroform-d) δ8.18–8.05(m,2H),7.67–7.55(m,2H),7.50(dd,J=5.3,2.0Hz,3H),7.31(t,J=8.0 Hz, 2H), 7.16(s...
Embodiment 136
[0098] Example 136: Preparation of (S)-pyrazolone chiral hydroxylation product Ib-1 (catalyst recycling)
[0099]
[0100]Weigh 5mmol pyrazolone Ia-1, add 2.5mol% phase transfer catalyst IIe-2, 0.005mmol TPP (porphyrin), put it into a 250mL reaction bottle, add 10% KOH aqueous solution 30mL, 100mL toluene, under 25W LED white light The reaction was stirred at -20 °C under irradiation. After reacting for 6 hours, the mixture was separated into layers, and the catalyst was slightly soluble in the organic layer and suspended in the water layer. The organic layer was collected, and the organic solvent was spin-dried, separated by column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain the asymmetric hydroxylated product Ib-1, and the ee was detected and the yield was calculated. Add 5 mmol of pyrazolone Ia-1, 0.005 mmol of TPP (porphyrin), and 100 mL of toluene to the water layer again, and continue stirring to react. After the reaction was finished, it was tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com