Method for producing zinc acrylate at low temperature
A technology of zinc acrylate and acrylic acid, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation and other directions, can solve the problems of low efficiency, high energy consumption for washing and recycling, poor performance of finished products, etc., to achieve low emission, protection Effectiveness, low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
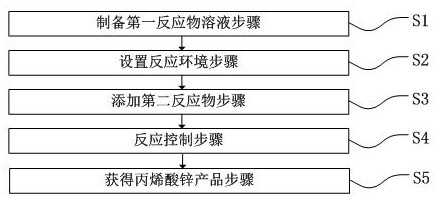
[0042] 800 to 1,000 kg of industrial grade acrylic acid, 100 to 250 kg of deionized water, mixed in the reactor and heated to 40 to 120 ° C, open the condensation reflux device, slowly add 400 to 550 kg of zinc oxide or basic zinc carbonate or hydrogen under stirring Zinc oxide or a mixture of two or three of the above substances, add 10 to 50 kg of hydrogen peroxide or acetic acid at the same time, and continue the reaction for 3 to 5 hours. When the material changes from a liquid-solid mixture to a mixture of solid powder and lumps, the reaction is over. , Dry the material, and when the moisture reaches the qualified requirements, the cooled material is pulverized to obtain zinc acrylate products.
Embodiment 2
[0044] 800 to 1000 kg of acrylic acid, 10 to 50 kg of acetic acid, 100 to 250 kg of deionized water, mixed in the reactor and heated to 40 to 120 ° C, open the condensation reflux device, slowly add 400 to 550 kg of zinc oxide or basic Zinc carbonate or zinc hydroxide or a mixture of two or three of the above substances, add 10 to 50 kg of hydrogen peroxide or deionized water at the same time, and continue to react for 3 to 5 hours. The material changes from a liquid-solid mixture to a solid powder and block. When the mixture is mixed, the reaction is over, the material is dried, and when the moisture reaches the qualified requirement, the cooled material is pulverized to obtain the zinc acrylate product.
Embodiment 3
[0046] 800 to 1000 kg of industrial grade acrylic acid, 10 to 20 kg of dilute hydrochloric acid, 100 to 250 kg of deionized water, mixed in the reactor and heated to 40 to 120 ° C, open the condensation reflux device, slowly add 400 to 550 kg of zinc oxide under stirring Or basic zinc carbonate or zinc hydroxide or a mixture of two to three of the above substances, add 10 to 50 kg of hydrogen peroxide or acetic acid at the same time, and continue the reaction for 3 to 5 hours. When the reaction is close to the end, add sodium hydroxide or potassium hydroxide or Ammonia solution neutralizes the residual acrylic acid. When the material changes from a liquid-solid mixture to a mixture of solid powder and lumps, the reaction is completed, and the material is dried. When the moisture reaches the qualified requirements, the cooled material is pulverized to obtain zinc acrylate products.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com