Synthesis process method of rosuvastatin
A technology for rosuvastatin and synthesis process, applied in the direction of organic chemistry, etc., can solve the problems of unfriendly process conditions, unsatisfactory E/Z selectivity and yield, and unsuitable for scale-up production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
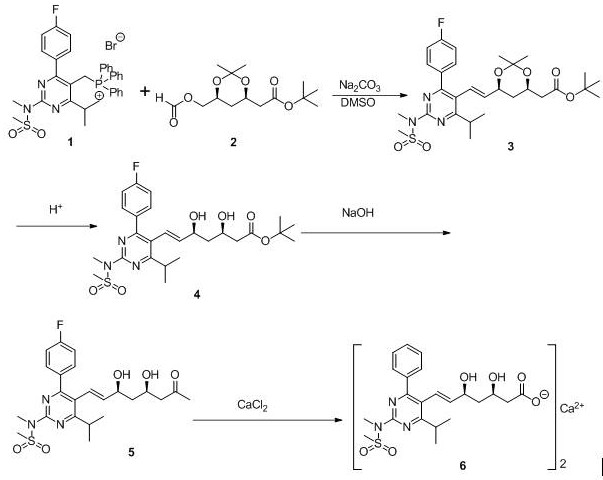
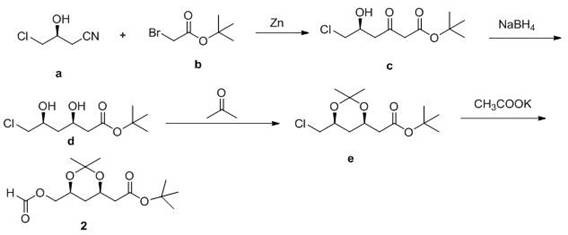
[0009] Embodiment 1: the synthesis of intermediate 2
[0010]
[0011] Under nitrogen protection, add 92 g of zinc powder into 800 mL of tetrahydrofuran, stir for 20 min, then add 69 g of a, slowly add 112.4 g of b at room temperature, raise the temperature to reflux for 3 h, slowly add 2 mol / L hydrochloric acid dropwise, and adjust the pH to 5-6, add 400 mL ethyl acetate and 400 mL water, separate the organic phase, extract the aqueous layer three times with 200 mL ethyl acetate, combine the organic phases, wash the organic phase with 200 mL saturated brine, and dry over anhydrous sodium sulfate , and then the solvent was removed by distillation under reduced pressure to obtain 101.2 g of oil c.
[0012] Dissolve 93.8 g of compound c in 1.3 L of dry tetrahydrofuran and 400 mL of ethanol, cool to -65°C under nitrogen protection, add 595 mL of sodium borohydride solution in tetrahydrofuran (1 mol / L), stir for 20 min, and then add boron Sodium hydride 22.6 g, react at this t...
Embodiment 2
[0015] Embodiment 2: the synthesis of intermediate 3
[0016] Add 6.78 g 1 (0.01mol), 3.02 g 2 (0.01mol), 30 mL dimethyl sulfoxide to the reaction flask, dissolve and stir, heat up to 70-80︒C, add 3.52 g sodium carbonate, stir overnight, TLC Monitor the response. After the reaction is completed, stop heating and cool to room temperature, slowly add 80 mL of water and 100 mL of toluene, stir and separate the phases, add toluene to the water phase for extraction (50 ml×2), combine the organic phases, add 60 mL of saturated saline to wash, 45︒C The solvent was removed under reduced pressure to obtain 5.2 g of a light yellow crude product, which was recrystallized from anhydrous methanol to obtain 5 g of product 3 as a white solid. The yield is 85%. The melting point is 145°C. m.p145°C. ESI-MS m / z: 590.3 [M+H] + . 1 HNMR (300MHz, DMSO- d 6 ), δ: 1.23~1.21 (d, J =6.66Hz, 6H), 1.58~1.79(m, 8H), 1.81~2.29(m, 2H), 2.42~2.51(d, J =13.44, 4.74 Hz, 2H), 3.28~3.39 (m, 1H), 2.95 ...
Embodiment 3
[0017] Embodiment 3: the synthesis of intermediate 4
[0018] Add 2.886 g (5 mmol) of intermediate 3 and 25 mL of acetonitrile into the reaction flask, dissolve and stir, then add 8 mL of 1 mol / L hydrochloric acid solution dropwise at 30-35 °C, keep warm and monitor the reaction by TLC. After the reaction, cool to room temperature, add 8 mL of 1 mol / L hydrochloric acid solution dropwise, and keep the reaction at 30-35°C; TLC monitors the reaction. After the reaction is complete, cool down to room temperature, add 3 mL of 1mol / L sodium hydroxide dropwise to maintain the pH at 9~10, then distill off acetonitrile at 45°C under reduced pressure, add 30 mL of water, and extract with ethyl acetate (10 mL×2) , combined the organic phases, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered with suction, and concentrated to dryness under reduced pressure to obtain 2.68 g of crude white solid. The crude product was purified with 20 mL of isopr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com