Multifunctional drug carrier material based on gold nanocage and preparation method thereof
A technology of gold nano-cages and carrier materials is applied in the fields of multifunctional drug carrier materials and their preparation, and the preparation of inorganic-organic hybrid drug carriers, which can solve the problem that drugs are difficult to penetrate into tumors, and achieve the effect of avoiding early release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Take SW1990 pancreatic cancer cell membrane / (gold nanocage-disulfide bond-gemcitabine / L-arginine) as an example.
[0062] 1) Synthesis of mercapto-polyethylene glycol-amino modified gold nanocages
[0063] Take 10 milliliters of ethylene glycol and heat it to 150 degrees Celsius in an oil bath under magnetic stirring, then add 0.12 milliliters of 3×10-3 mol / liter of sodium hydrosulfide in ethylene glycol) into the solution quickly. After 4 minutes, 1 mL of hydrogen chloride solution (prepared by adding 4 µl of 12 mol / L hydrochloric acid to 12 mL of ethylene glycol) was added to the mixture, followed by 2.5 mL of polyvinylpyrrolidone in ethylene glycol after 2 minutes. solution (prepared by dissolving 60 mg of polyvinylpyrrolidone in 3 ml of ethylene glycol). After another 2 minutes, 0.8 ml of 282×10-3 mol / L silver trifluoroacetate ethylene glycol solution was added to the mixed solution, and the reaction was continued for 1 hour at 150 degrees Celsius, washe...
example 1
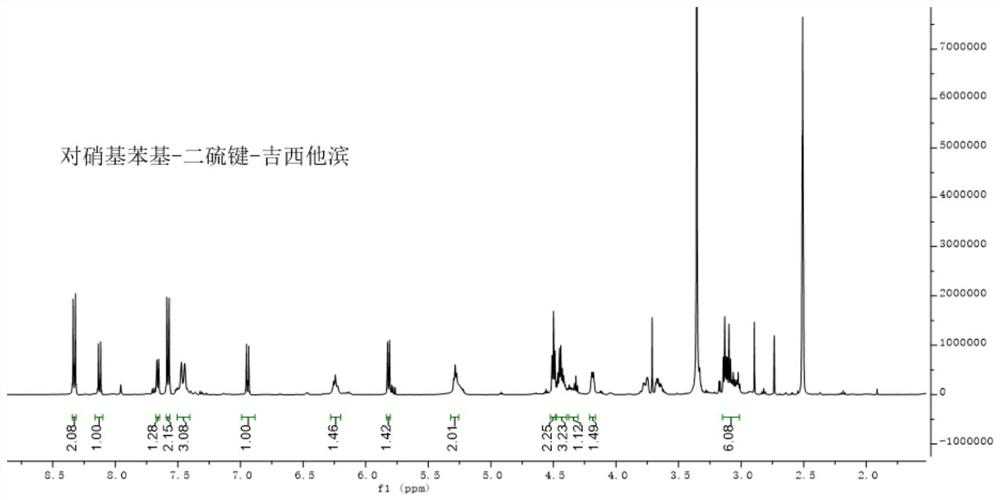
[0074] The proton nuclear magnetic resonance spectrum of p-nitrophenyl-disulfide bond-gemcitabine in example 1 is as figure 1 As shown, the figure fully demonstrates the feasibility of the compound preparation.
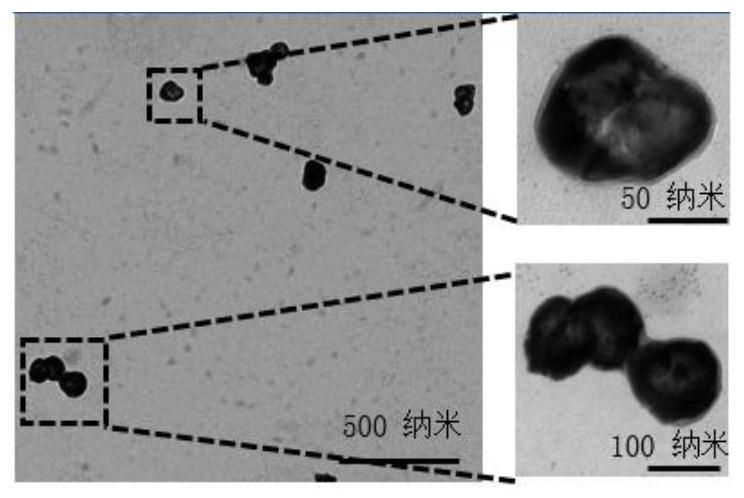
[0075] The transmission electron micrograph of SW1990 pancreatic cancer cell membrane / (gold nanocage-disulfide bond-gemcitabine / L-arginine) in example 1 is as figure 2 Shown, fully proved the feasibility of the preparation method of the present invention.
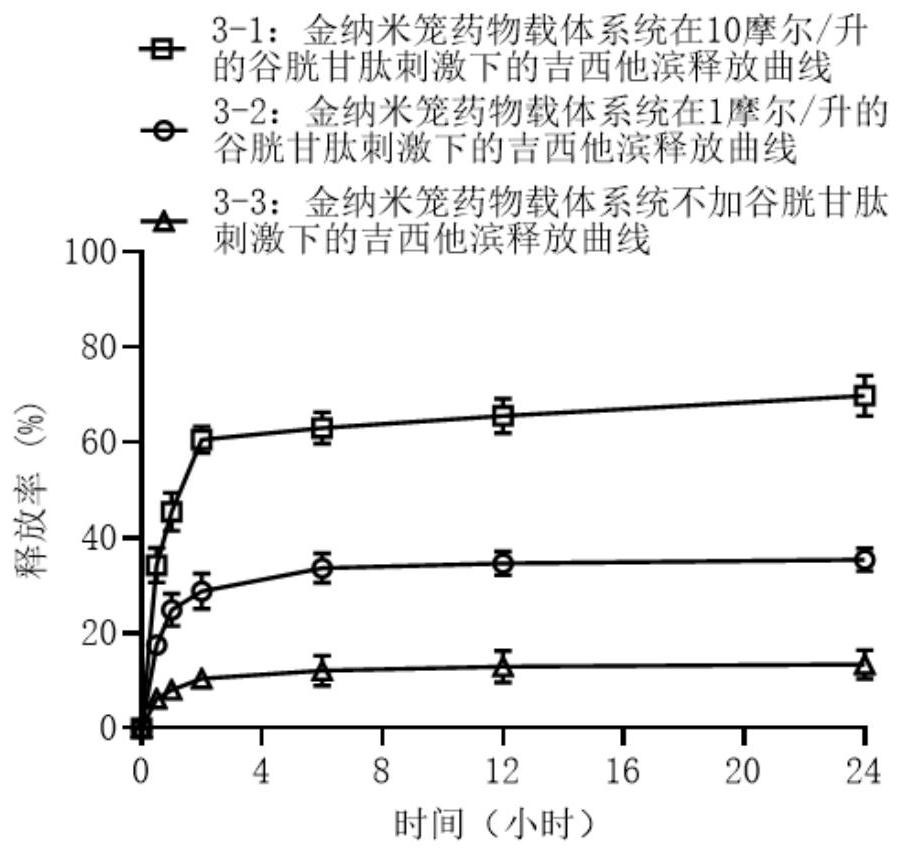
[0076] The gemcitabine release pattern of SW1990 pancreatic cancer cell membrane / (gold nanocage-disulfide bond-gemcitabine / L-arginine) in example 1 under redox stimulation is as follows image 3 As shown, it fully proves that the gold nanocage drug carrier system of the present invention can release the drug gemcitabine under redox stimulation.
[0077] The nitric oxide release pattern of SW1990 pancreatic cancer cell membrane / (gold nanocage-disulfide bond-gemcitabine / L-arginine) under the action of active oxyge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com