Preparation method of (22E, 24R)-3alpha, 5-cyclo-5alpha-ergosta-7, 22-diene-6-ketone
A technology for ergosterol and ergosterol, applied in the field of -3α, can solve the problems of high operator requirements, serious environmental pollution, potential safety hazards, etc., and achieve the effects of simple post-processing process, high yield and improved safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
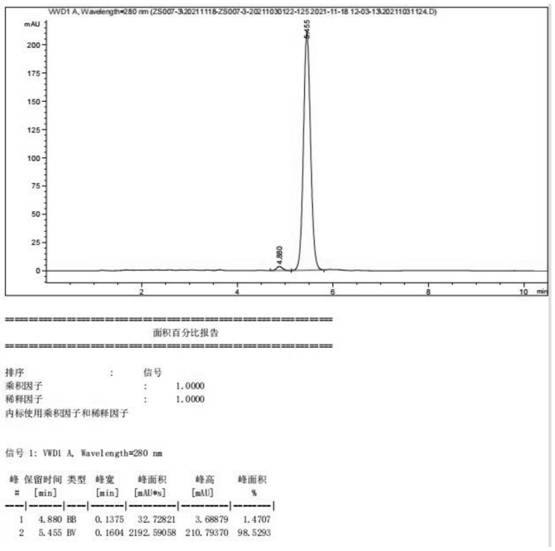
Embodiment 1
[0043] Preparation of (22E, 24R) -3α, 5-ring-5α-Wheat-7,22-diene-6-ketone, including the following steps:
[0044] 1) Add 100.0 g of maltolosterol to the reaction bottle, 1000.0 g of triethylamine, 38.3 g of triethylamine, stirred for 0.5 h, control temperature 20 ° C, add methanesulfonyl chloride 43.3g, drop 0.5 h, after 5 Continue at 20 ° C insulation reaction 2H, TLC tracking (ethyl acetate: petroleum ether = 1: 8, volume ratio) The reaction ended, 0.5 h, 0.5 h, dripping, and stirred at 10 min, stop After stirring, it plas with 0.5 h, phase phase, and the lower water phase wastewater treatment, the upper organic phase is a butanone solution containing the first step product, and the next reaction is carried out without treatment.
[0045] 2) Tutinone solution (yield) containing the first step product (yield according to 100% meter) to the step 1), 50.4 g of potassium hydrogencarbonate, stirring heating to 60 ° C and heat preheating (acetic acid) Ethyl ester: petroleum ether = 1...
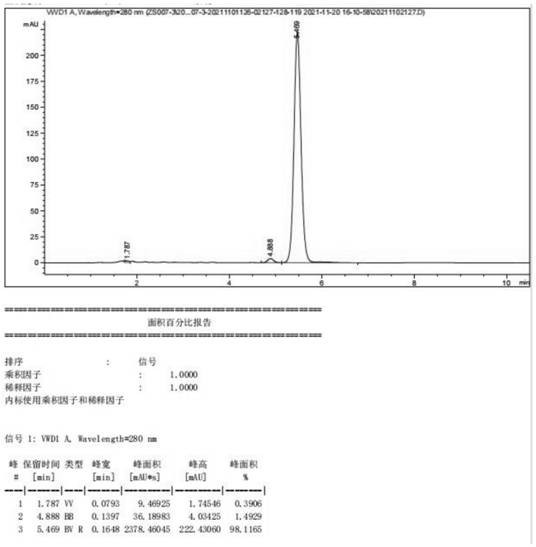
Embodiment 2
[0050] Preparation of (22E, 24R) -3α, 5-ring-5α-Wheat-7,22-diene-6-ketone, including the following steps:
[0051] 1) Add 100.0 g of maltolosterol 100.0 g, butanone 1200.0 g and triethylamine 51.1 g, stirred for 0.5 h, temperature control to 10 ° C, add methanesulfonyl chloride 57.7., 0.5 h, and add 0.5 hours The reaction was continued at 10 ° C for 3 h, TLC tracking (ethyl acetate: petroleum ether = 1: 8, volume ratio) ended, adding 600 g of ice water to the reaction, adding 1 h, dripping, adding 40 min, stopping After stirring, it is allowed to stand for 1 h, phase phase, the lower water phase is taken as a wastewater treatment, and the upper organic phase is a butanone solution containing the first step product, and the next reaction is carried out directly;
[0052] 2) Tutinone solution (yield according to 100% meter) obtained from step 1), 75.6 g of potassium hydrogencarbonate, stirring heating temperature to 70 ° C and heat preheating for 3 h, TLC tracking (acetic acid) Ethy...
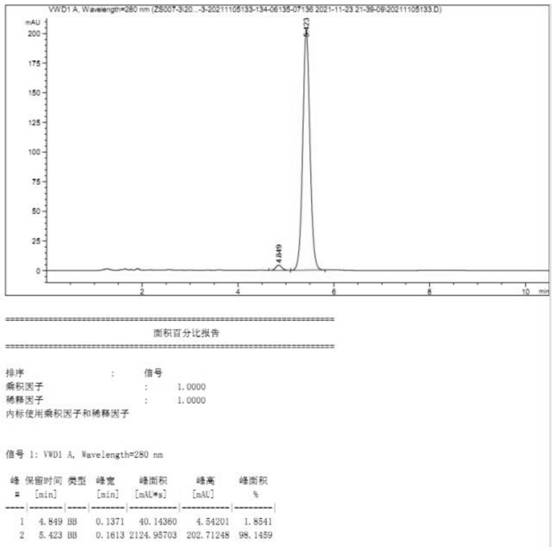
Embodiment 3
[0056] Preparation of (22E, 24R) -3α, 5-ring-5α-Wheat-7,22-diene-6-ketone, including the following steps:
[0057] 1) Add 100.0 g of maloleol 100.0 g, butanone 1500.0 g and triethylamine 76.6 g, stirring 0.5 h, controlled temperature at 0 ° C, titanalesulfonyl chloride 86.6 g, drop 0.5 h, after drying The reaction was continued at 0 ° C for 4 h, TLC tracked (ethyl acetate: petroleum ether = 1: 8, volume ratio), 0. The ice water is quenched, dripping 2 h, dripping, adding 60 min, stop stirring After standing for 2H, phase phase, lower water phase wastewater treatment, the upper organic phase is a butanone solution containing the first step product, and the next reaction is carried out without treatment;
[0058] 2) Tutinone solution containing the first step product (yield according to 100% meter) is added to the step 1), 100.8.8 g of potassium hydrogencarbonate, stirred by stirring to 80 ° C and heat it up (acetic acid) Ethyl ester: petroleum ether = 1: 8, volume ratio) The reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com