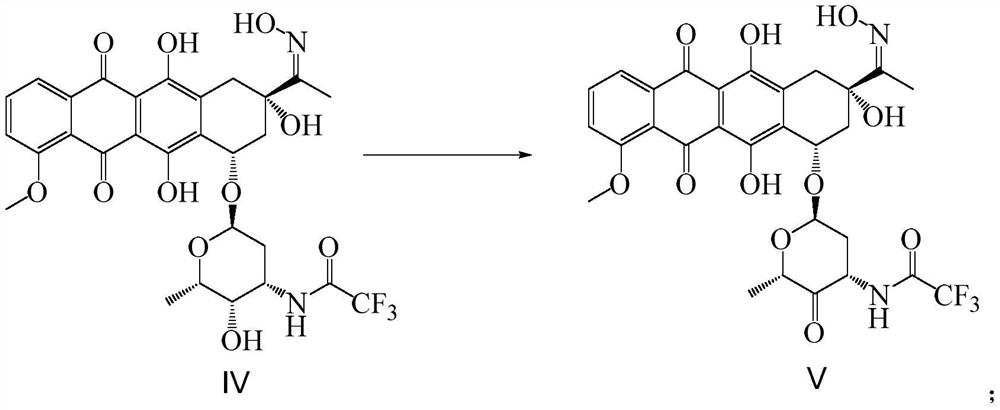
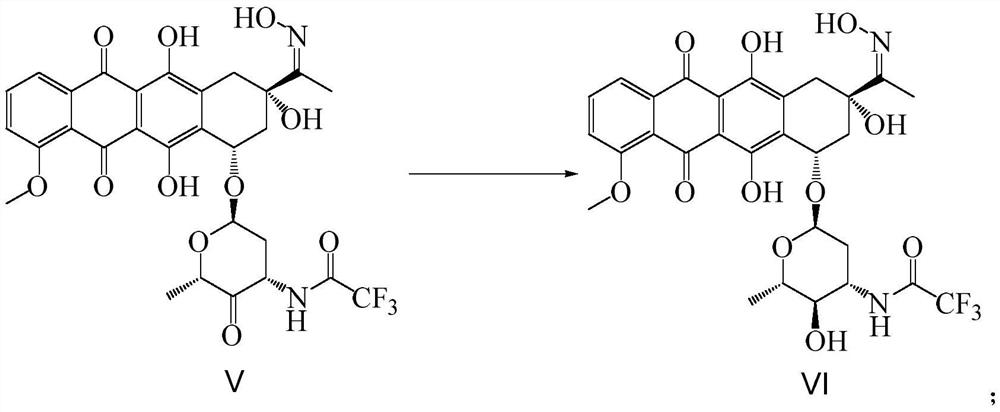
Synthetic method of epirubicin hydrochloride and intermediate of epirubicin hydrochloride
A technology of epirubicin hydrochloride and synthetic methods, applied in the direction of organic chemical methods, chemical instruments and methods, sugar derivatives, etc., can solve the problems of yield improvement, low tolerance, low purity, etc., and achieve operational steps Simple and easy to implement, expanded reduction methods, cheap and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
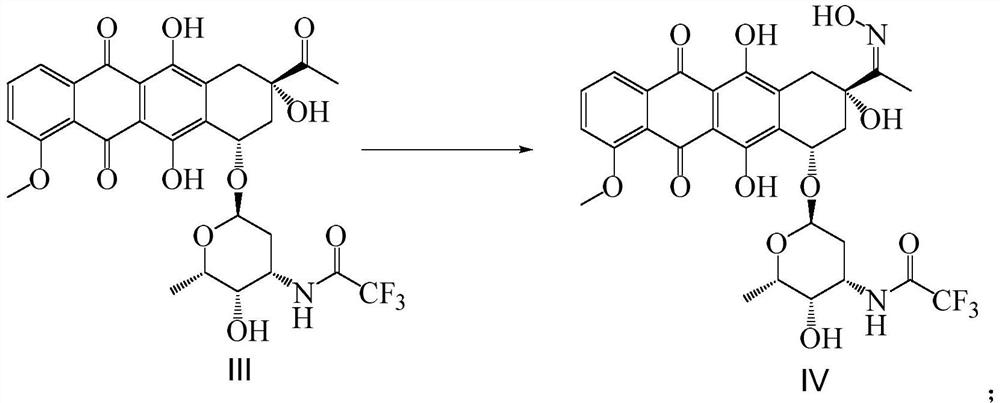
[0078] Synthesis of Intermediate Compound IV:
[0079] Add 10.00g of compound III (16.04mmol) and 500mL of methanol into a 1L three-necked flask, stir to dissolve, dissolve 1.46g (20.85mmol) of hydroxylamine hydrochloride in 6mL of water, and adjust the pH to 8 with 6mol / L NaOH aqueous solution, and dissolve the hydrochloric acid The aqueous solution of hydroxylamine was added to the methanol solution of compound III, and the temperature was raised to 60°C. After stirring for about 2 hours, the reaction progress was detected by HPLC. After the reaction was completed, the temperature was lowered to room temperature, and chloroform was added to the reaction solution with an equal volume of methanol. The liquid was separated, and the organic phase was concentrated under reduced pressure until no liquid flowed out to obtain the intermediate compound IV (9.90 g, yield 96.7%, purity 95.9%) in the form of a red foamy solid. The HPLC chromatographic conditions of compound IV are: chro...
Embodiment 2
[0081] Synthesis of Intermediate Compound IV:
[0082] Add 10.00g of compound III (16.04mmol) and 400mL of ethanol to a 1L three-necked flask, stir to dissolve, dissolve 2.23g (32.08mmol) of hydroxylamine hydrochloride in 11.2mL of water, and adjust the pH to 8 with 6moL / L NaOH aqueous solution. Add the aqueous solution of hydroxylamine hydrochloride to the methanol solution of Compound III, raise the temperature to 55°C, stir and react for about 2 hours, use HPLC to detect the reaction progress, after the reaction is completed, cool down to room temperature, and add chloroform with the same volume as ethanol to the reaction solution , liquid separation, the organic phase was concentrated under reduced pressure until no liquid flowed out to obtain the intermediate compound IV (9.84g, yield 96.1%, purity 95.7%) of red foamy solid, the HPLC chromatographic conditions of compound IV were the same as in Example 1 .
Embodiment 3
[0084] Synthesis of Intermediate Compound IV:
[0085] Add 10.00g of compound III (16.04mmol) and 800mL of ethanol to a 1L three-necked flask, stir to dissolve, dissolve 1.11g (16.04mmol) of hydroxylamine hydrochloride in 3.3mL of water, and adjust the pH to 9 with 6moL / L NaOH aqueous solution. Add the aqueous solution of hydroxylamine hydrochloride to the methanol solution of Compound III, raise the temperature to 62°C, stir and react for about 2 hours, use HPLC to detect the reaction progress, after the reaction is completed, cool down to room temperature, and add chloroform with an equal volume of ethanol to the reaction solution , liquid separation, the organic phase was concentrated under reduced pressure until no liquid flowed out to obtain the intermediate compound IV (9.75g, yield 95.2%, purity 94.9%) of the red foamy solid, and the HPLC chromatographic conditions of the compound IV were the same as in Example 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com