Difructose anhydride hydrolase mutant E160F with improved thermal stability
A double fructose anhydrolase technology, applied in the field of enzyme engineering, can solve the problems of low activity of double fructose anhydrolase, poor thermal stability, and limited property research, and achieve the effect of broadening industrial applications and solving poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation and Enzyme Activity Determination of Wild Type Double Fructose Anhydrolase
[0031] The genome number of the microbial Arthrobacter chlorophenolicus A6 strain in GenBank is CP001341, which contains a double fructoanhydrolase gene (number Achl_2895), and the full length of the gene is 1338 nucleotides (SEQ ID NO.2). The protease number coded by the gene is ACL40859.1, with a total of 445 amino acids (SEQ ID NO.1).
[0032] 1) Construction of recombinant strains:
[0033] The gene encoding double fructoanhydrolase is molecularly cloned and expressed in Escherichia coli engineering bacteria, and the nucleotide sequence such as SEQ ID NO.2 double fructoanhydrolase gene is constructed to the Xho I of the plasmid vector pET-22b (+) Between the Nde I restriction site, a recombinant plasmid was constructed and named pET-22b(+)-AcDFA-IIIase.
[0034] Transform the plasmid pET-22b(+)-AcDFA-IIIase into E.coli BL21(DE3) competent cells in a medium containin...
Embodiment 2
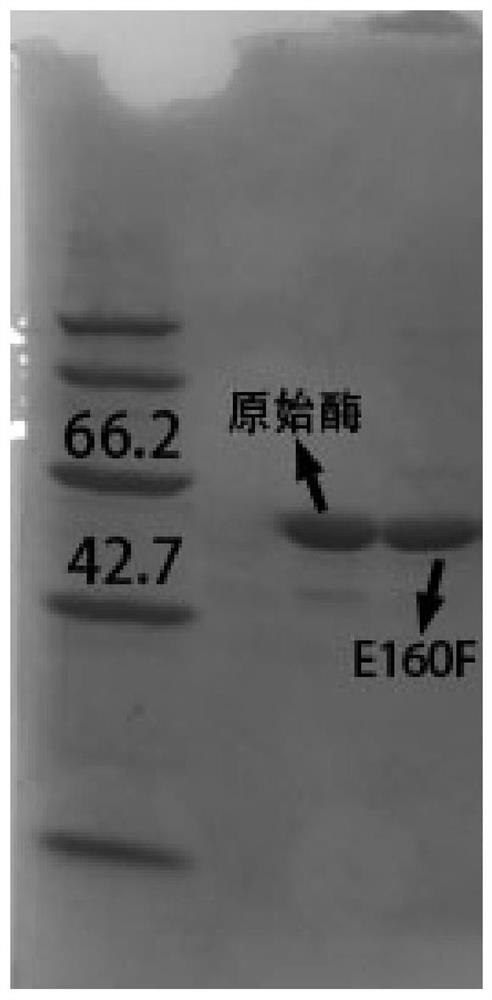
[0041] Example 2: Preparation and expression purification of double fructoanhydrolase mutant E160F
[0042] (1) Preparation of mutant E160F
[0043] The nucleotide sequence such as SEQ ID NO.2 double fructoanhydrolase gene is used as a template for primer design, and the carrier pET-22b(+)-AcDFA- IIIase was used as template for site-directed mutagenesis to construct mutant plasmid pET-22b(+)-E160F.
[0044] Mutant Forward Primer: 5'-GCGGTCGCCATCCG TTC AATACCTA-3'
[0045] Mutant reverse primer: 5'-ATTGGCATAGGTATT GAA CGGATGGCGA-3'
[0046] The PCR reaction system is: 10×PCR Buffer 5 μL, dNTP (2 mmol / L) 4 μL, mutant forward primer (10 μM) 1 μL, mutant reverse primer (10 μM) 1 μL, template pET-22b(+)-AcDFA-IIIase 1 μL, Taq Plus DNA polymerase (5U / μL) 0.5 μL, use double distilled water to make up the volume of the reaction system to 50 μL.
[0047] PCR amplification conditions were: pre-denaturation at 94°C for 4 min; denaturation at 94°C for 1 min, annealing at 56°C for 1 ...
Embodiment 3
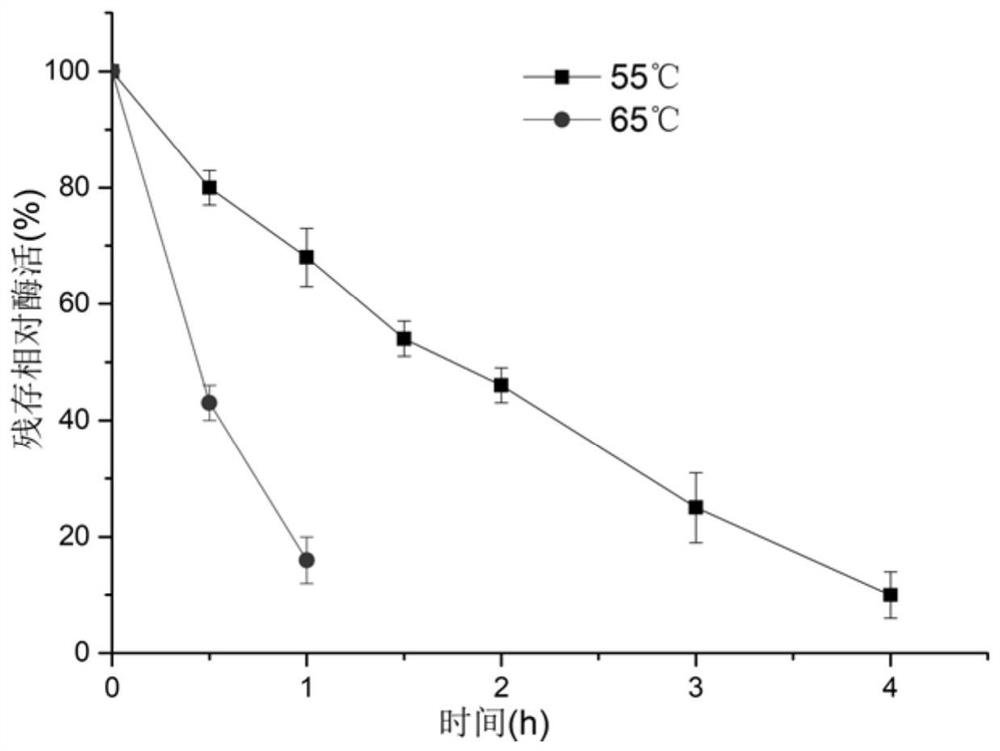
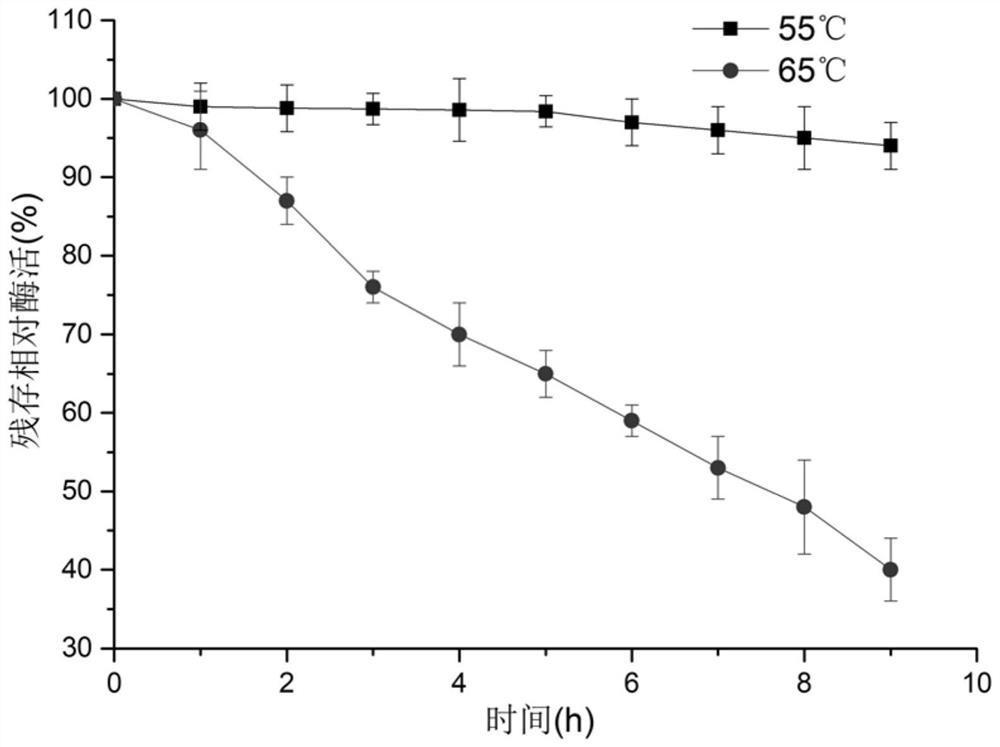
[0056] Embodiment 3: Enzyme thermostability assay
[0057] (1) Detection of optimum temperature:
[0058] In a 1mL reaction system, include a final concentration of 10g L -1 Substrate bisfructose anhydride, pH 6.5 phosphate buffer at a final concentration of 50 mM and 100 nmol L -1 Pure enzyme solution was reacted at 30°C, 40°C, 45°C, 50°C, 55°C, 60°C, 65°C, 70°C, and 80°C for 10 minutes, and the reaction was terminated in a boiling water bath for 10 minutes. After centrifugation at 18000×g for 20min at 4°C, the reaction solution was filtered through a 0.22 μm filter membrane into a liquid phase vial, and the high-performance liquid phase was equipped with a sugar column Sugar-Pak I (4.6mm×250mm, Waters, MA, USA) and a differential refraction display. for the detection of inulin. 1U of enzyme activity is defined as the amount of enzyme required to produce 1 μmol of inulin per minute at pH 6.5 and 55°C.
[0059] The results showed that the optimal catalytic temperature of d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com