Fluorescent probe for detecting zinc ions as well as preparation method and application of fluorescent probe
A fluorescent probe, zinc ion technology, applied in the field of fluorescent probes, to achieve the effects of low cost, high improvement cost and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of a fluorescent probe for detecting zinc ions:
[0038] (1) Take 3,8 dibromo-10-phenanthroline-5,6-dione (0.75g, 2.0475mmol), ammonium acetate (2.3625g, 60mmol), p-hydroxybenzaldehyde (0.2497g, 2.0475mmol) Dissolve in glacial acetic acid (26.25ml), keep at 90°C for two hours, then increase the temperature to 120°C for reaction, stop the reaction, add water (200ml) to dilute, adjust the pH to neutral with concentrated ammonia, and filter to obtain the crude product Compound 1 was obtained by purification on a silica gel chromatography column (developing solvent: ethanol: dimethyl sulfoxide = 40: 1).
[0039] (2) Dissolve compound 1 (0.1753g, 0.3mmol) and tetraphenylethylene borate (0.1410g, 0.3mmol) prepared in step (1) in DMF (10ml), then add the catalyst tetrakis (triphenyl Phosphine) palladium (4mg), 2M K 2 CO 3 (2ml) solution, the temperature was maintained at 120 degrees Celsius under the protection of nitrogen, and the reaction was carried ...
Embodiment 2
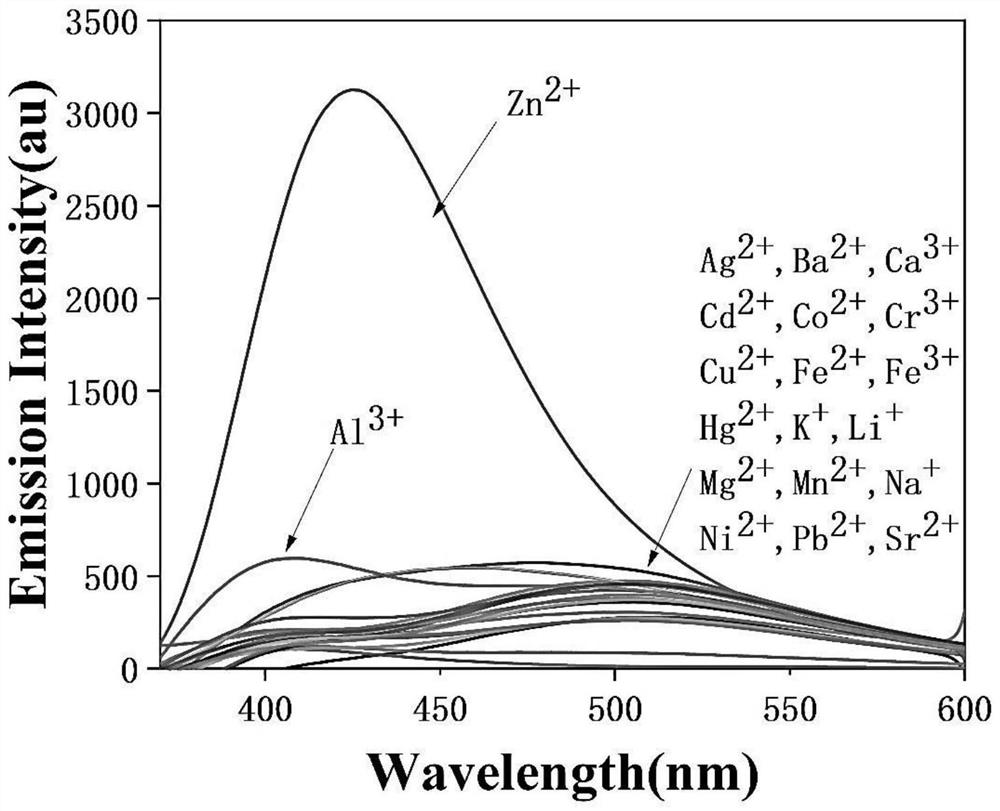
[0041] Ion selectivity tests for target products, such as figure 1 . P and metal ions were added to the water-ethanol solution (1:1, v / v), and the concentration of P in the test solution was 1×10 -6 mol / L, add 5 times the equivalent of sodium ions (Na + ), magnesium ions (Mg 2 + ), calcium ions (Ca 2+ ), cadmium ions (Cd 2+ ), chromium ions (Cr 2+ ), lithium ion (Li + ), barium ion (Ba 2+ ), manganese ions (Mn 2+ ), potassium ions (K + ), ferrous ions (Fe 2+ ), ferric ions (Fe 3+ ), cobalt ions (Co 2+ ), nickel ions (Ni 2+ ), copper ions (Cu 2+ ), cesium ions (Sr 2+ ), aluminum ions (Al 2+ ). It can be seen from the figure that the emission peaks of the system are basically unchanged except for zinc ions. After adding zinc ions, the fluorescence intensity is significantly enhanced (about 15 times), accompanied by an obvious blue shift. It shows that the fluorescent probe P has better fluorescence selective recognition ability for zinc ions.
Embodiment 3
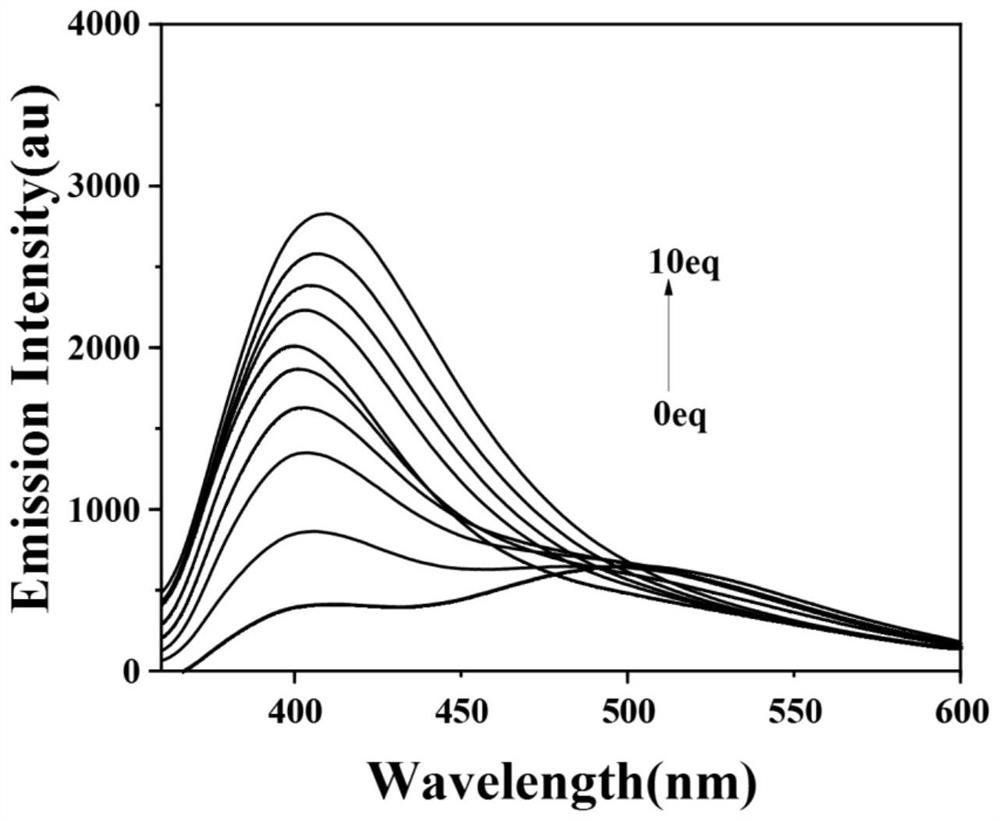
[0043] Fluorescent titration test of target compounds against zinc ions, such as figure 2 . Dissolve P in ethanol to a concentration of 1×10 -6 mol / L standard solution, then dropwise add zinc ion solution with a molar ratio of 0-10eq, and test the fluorescence spectrum at room temperature. From the test results, it can be found that after adding 0.001 times the equivalent of zinc ions, the fluorescence emission peaks are significantly enhanced and blue-shifted. And with the increase of zinc ion concentration in the test system, the fluorescence emission intensity also increases. This indicates that the fluorescent probe P has a high sensitivity for the recognition of zinc ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com